Abstract
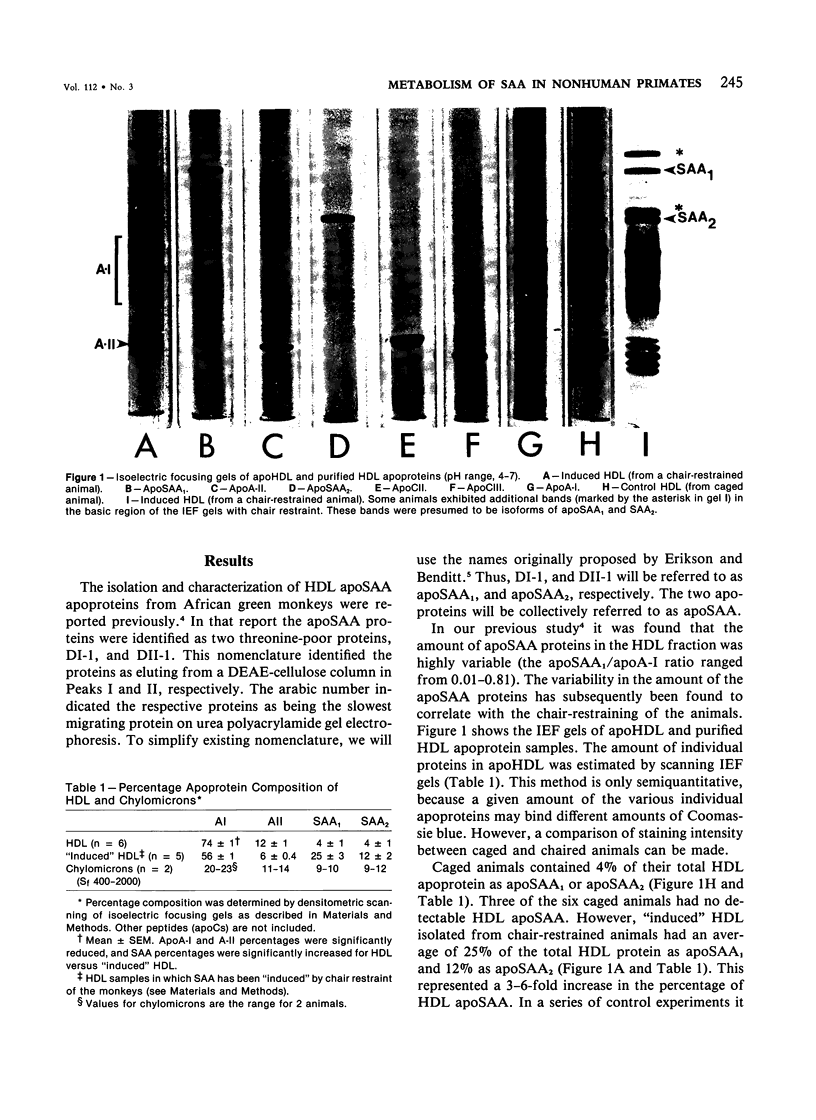
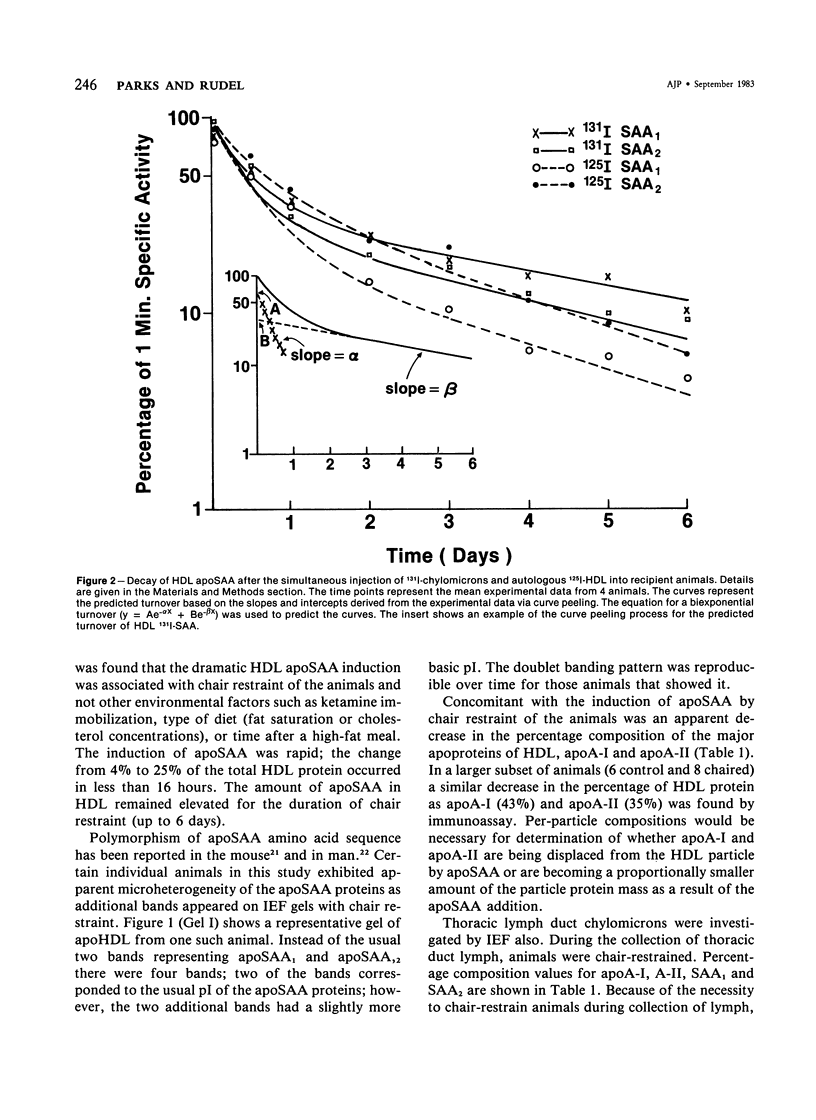
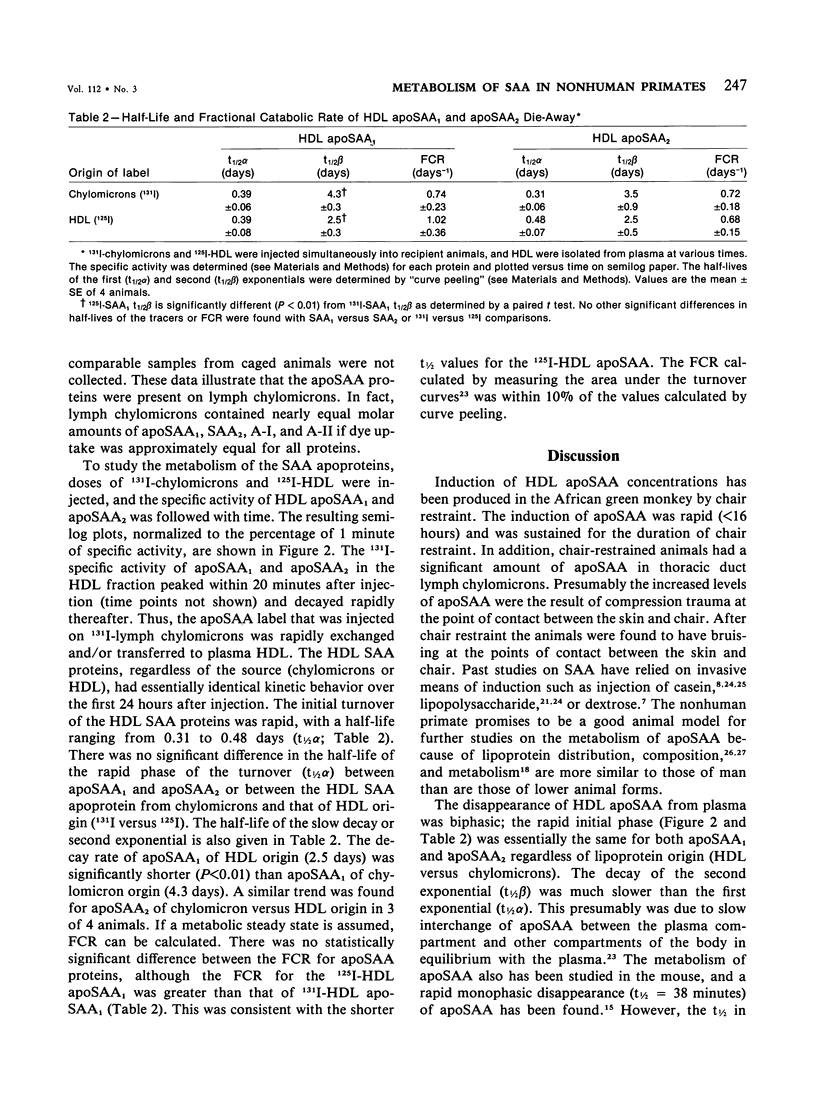
Serum amyloid protein (SAA) has been reported to be an apoprotein of high-density lipoprotein (HDL), but little is known concerning its metabolism. In this study, apoSAA was induced in nonhuman primate plasma HDL and thoracic duct lymph chylomicra by overnight chair restraint of the animals. There was a 3-6-fold increase in plasma HDL apoSAA in chair-restrained animals when compared with caged (control) animals. Lymph chylomicrons of chaired animals also contained significant amounts (approximately 20% of total protein) of apoSAA. For study of the metabolism of HDL apoSAA, animals were given injections of 131I-labeled lymph chylomicrons and autologous 125I-HDL. HDL were isolated from the plasma of recipient animals between 1 minute and 5 days after injection, and the specific activity of the apoSAA was determined. The turnover of apoSAA from plasma was biphasic, the initial phase having a t1/2 (0.39-0.48 days) similar regardless of the source (chylomicrons versus HDL) of the injected dose. The second phase of the turnover was significantly faster (t1/2 = 2.5 days) for apoSAA of 125I-HDL origin than that of 131I-chylomicron origin (t1/2 = 4.3 days). This difference also was suggested by the fractional catabolic rates (FCR) of 125I-HDL and 131I-chylomicron apoSAA (1.02 versus 0.74 d-1, respectively). From these studies it was concluded that 1) apoSAA can be rapidly induced in plasma HDL and lymph chylomicrons of nonhuman primates by chair restraint; 2) HDL apoSAA is catabolized more rapidly than HDL apoA-I and apoA-II; 3) and the catabolic rate of HDL apoSAA may be determined, in part, by its lipoprotein origin (chylomicrons versus HDL).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumal R., Sklar S., Wilson B., Laskov R. Casein-induced murine amyloidosis: amyloidogenesis in vitro by monolayer spleen explants of casein-injected mice. Lab Invest. 1978 Dec;39(6):632–639. [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Kleiner E. Synthesis and secretion of serum amyloid protein A (SAA) by hepatocytes in mice treated with casein. J Immunol. 1980 Feb;124(2):495–499. [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Levo Y., Frangione B., Franklin E. C. Polymorphism of tissue and serum amyloid A (AA and SAA) proteins in the mouse. J Immunol. 1978 Jul;121(1):138–140. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Jacob M. P., Bellon G., Robert L., Hornebeck W., Ayrault-Jarrier M., Burdin J., Polonovski J. Elastase-type activity associated with high density lipoproteins in human serum. Biochem Biophys Res Commun. 1981 Nov 16;103(1):311–318. doi: 10.1016/0006-291x(81)91694-6. [DOI] [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978 Oct 1;148(4):1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Malmendier C. L., Ameryckx J. P. S-peptides--new apoproteins of human high density lipoproteins induced by glucose infusions. Clin Chim Acta. 1981 Apr 9;111(2-3):267–270. doi: 10.1016/0009-8981(81)90194-7. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Elin R. J., Sipe J. D., Wolff S. M. Changes in human serum amyloid A and C-reactive protein after etiocholanolone-induced inflammation. J Clin Invest. 1978 Feb;61(2):390–394. doi: 10.1172/JCI108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam K. P., Sipe J. D. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med. 1976 Oct 1;144(4):1121–1127. doi: 10.1084/jem.144.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior G. W., Rudel L. L. Heterogeneity in the low density lipoproteins of cholesterol fed African green monkeys (Cercopithecus aethiops). Biochim Biophys Acta. 1978 Dec 22;531(3):331–343. doi: 10.1016/0005-2760(78)90215-1. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Parks J. S., Rudel L. L. Different kinetic fates of apolipoproteins A-I and A-II from lymph chylomicra of nonhuman primates. Effect of saturated versus polyunsaturated dietary fat. J Lipid Res. 1982 Mar;23(3):410–421. [PubMed] [Google Scholar]
- Parks J. S., Rudel L. L. Isolation and characterization of high density lipoprotein apoproteins in the non-human primate (vervet). J Biol Chem. 1979 Jul 25;254(14):6716–6723. [PubMed] [Google Scholar]
- Patsch J. R., Gotto A. M., Jr, Olivercrona T., Eisenberg S. Formation of high density lipoprotein2-like particles during lipolysis of very low density lipoproteins in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4519–4523. doi: 10.1073/pnas.75.9.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C., Frangione B., Greenspan J. Isolation and partial characterization of SAA-an amyloid-related protein from human serum. J Immunol. 1976 May;116(5):1415–1418. [PubMed] [Google Scholar]
- Rosenthal C. J., Sullivan L. Serum amyloid A: evidence for its origin in polymorphonuclear leukocytes. J Clin Invest. 1978 Dec;62(6):1181–1186. doi: 10.1172/JCI109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Reynolds J. A., Bullock B. C. Nutritional effects on blood lipid and HDL cholesterol concentrations in two subspecies of African green monkeys (Cercopithecus aethiops). J Lipid Res. 1981 Feb;22(2):278–286. [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Lewis S. B. Isolation and characterization of two threonine-poor apolipoproteins of human plasma high density lipoproteins. Biochemistry. 1978 May 30;17(11):2174–2179. doi: 10.1021/bi00604a023. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]



