Abstract
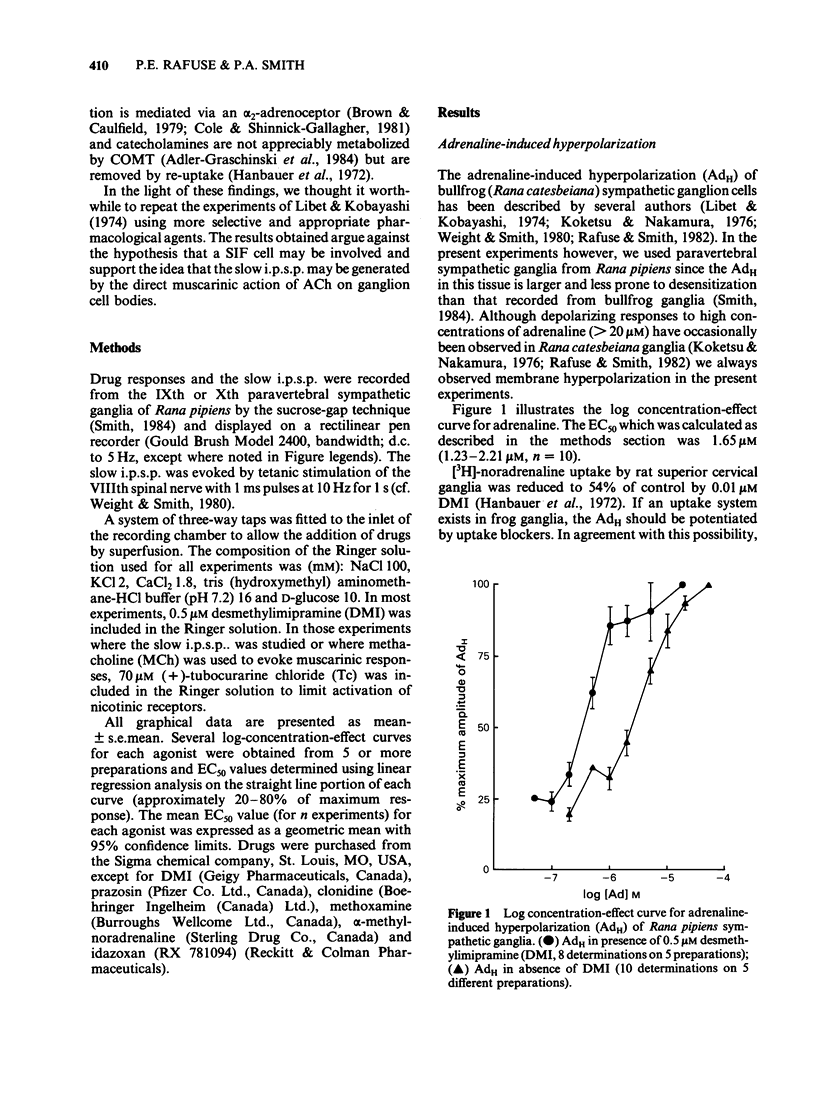
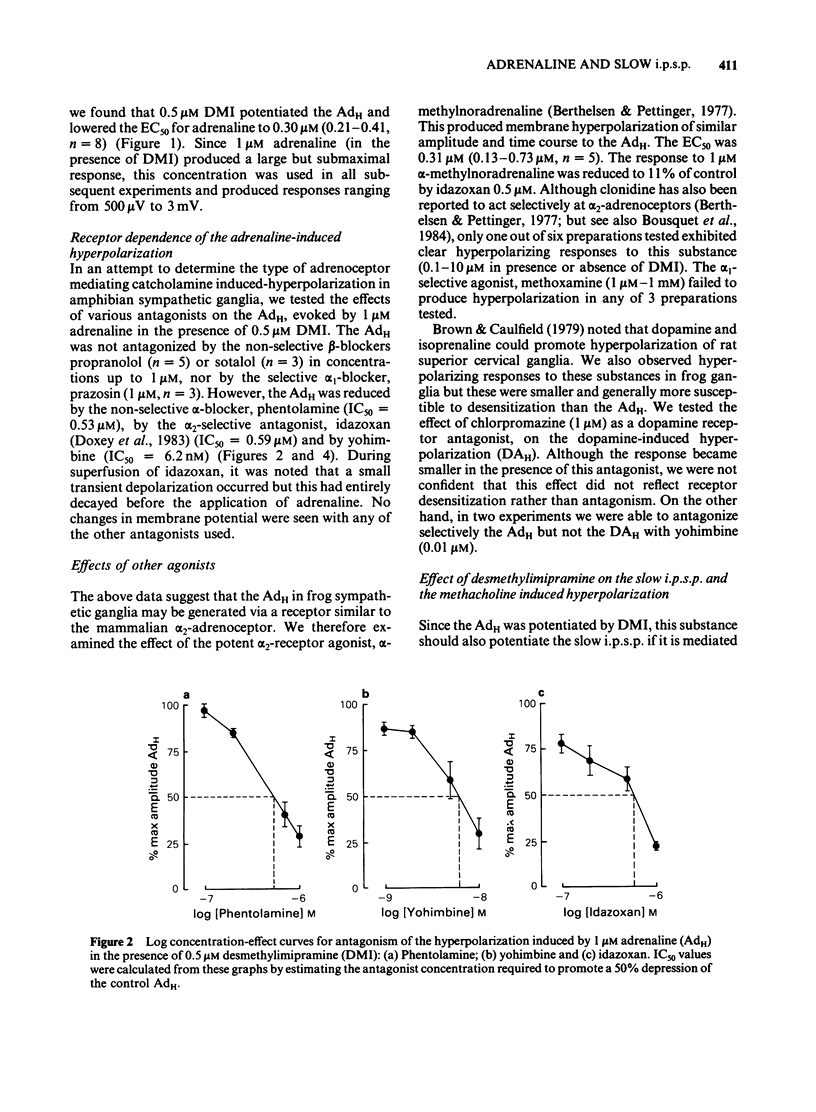
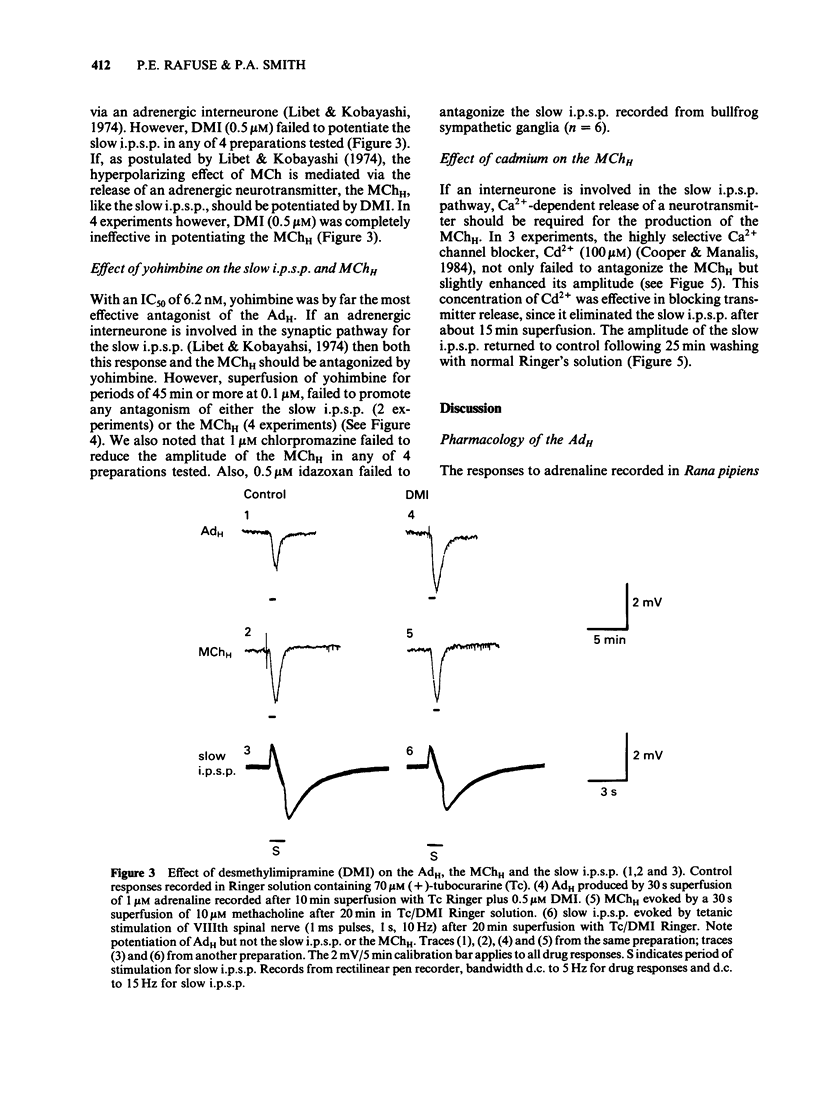
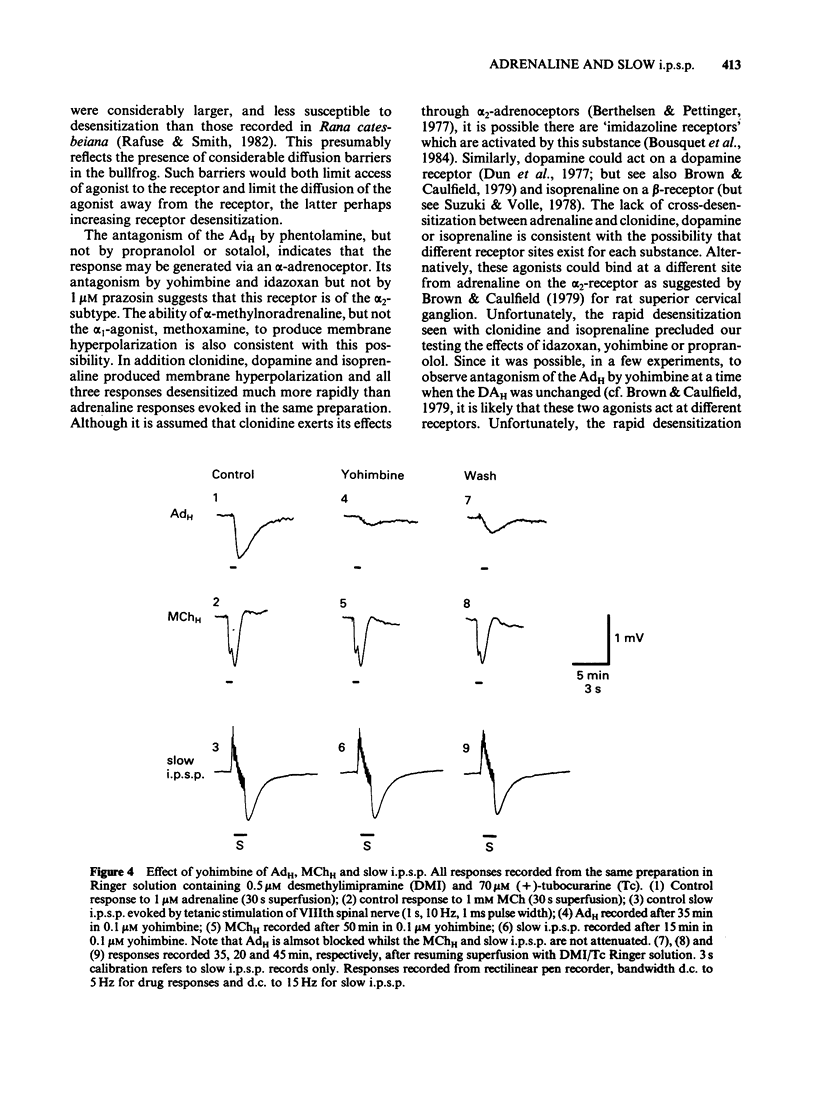
The adrenaline-induced hyperpolarization (AdH), slow inhibitory postsynaptic potential (slow i.p.s.p.) and hyperpolarizing phase of the response to methacholine (MChH) in Rana pipiens sympathetic ganglia were studied by means of the sucrose-gap technique. Desmethylimipramine (DMI, 0.5 microM) lowered the EC50 for adrenaline from 1.65 microM (1.23-2.21 microM, n = 10) to 0.30 microM (0.21-0.41 microM, n = 8). DMI did not potentiate the slow i.p.s.p. or the MChH. Propranolol, sotalol or prazosin (1 microM) did not antagonize the AdH. The response was antagonised by phentolamine (IC50 = 0.53 microM), yohimbine (IC50 = 6.2 nM) and idazoxan (IC50 = 0.59 microM). Yohimbine (0.1 microM) did not reduce the amplitude of the slow i.p.s.p. or the MChH. The slow i.p.s.p. was eliminated in Ringer solution containing Cd2+ (100 microM). This concentration of Cd2+ did not reduce the amplitude of the MChH. Alpha-Methylnoradrenaline produced a concentration-dependent hyperpolarization with an EC50 of 0.31 microM (0.13-0.73 microM, n = 5), in the presence of DMI (0.5 microM). These results are consistent with the hypothesis that the AdH may be generated by activation of a receptor similar to the mammalian alpha 2-adrenoceptor. No evidence was found in support of the hypothesis that an adrenergic interneurone is involved in the synaptic pathway for the slow i.p.s.p.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol. 1982 Nov;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler-Graschinsky E., Filinger E. J., Martínez A. E. Ionic mechanisms involved in the release of 3H-norepinephrine from the cat superior cervical ganglion. Life Sci. 1984 Feb 27;34(9):861–871. doi: 10.1016/0024-3205(84)90203-0. [DOI] [PubMed] [Google Scholar]
- Ashe J. H., Libet B. Pharmacological properties and monoaminergic mediation of the slow IPSP, in mammalian sympathetic ganglion. Brain Res. 1982 Jun 24;242(2):345–349. doi: 10.1016/0006-8993(82)90321-3. [DOI] [PubMed] [Google Scholar]
- Atlas D., Adler M. alpha-adrenergic antagonists as possible calcium channel inhibitors. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1237–1241. doi: 10.1073/pnas.78.2.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Bousquet P., Feldman J., Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984 Jul;230(1):232–236. [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Alpha-adrenoceptor and dopamine receptor antagonists do not block the slow inhibitory postsynaptic potential in sympathetic ganglia. Brain Res. 1980 Apr 7;187(1):226–230. doi: 10.1016/0006-8993(80)90510-7. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Comparison of the receptors mediating the catecholamine hyperpolarization and slow inhibitory postsynaptic potential in sympathetic ganglia. J Pharmacol Exp Ther. 1981 May;217(2):440–444. [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Muscarinic inhibitory transmission in mammalian sympathetic ganglia mediated by increased potassium conductance. Nature. 1984 Jan 19;307(5948):270–271. doi: 10.1038/307270a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. P., Manalis R. S. Cadmium: effects on transmitter release at the frog neuromuscular junction. Eur J Pharmacol. 1984 Apr 6;99(4):251–256. doi: 10.1016/0014-2999(84)90131-6. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J. Ganglionic transmission: electrophysiology and pharmacology. Fed Proc. 1980 Oct;39(12):2982–2989. [PubMed] [Google Scholar]
- Dun N. J., Kaibara K., Karczmar A. G. Dopamine and adenosine 3',5'-monophosphate responses of single mammalian sympathetic neurons. Science. 1977 Aug 19;197(4305):778–780. doi: 10.1126/science.196332. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. A comparative study of the pharmacological properties of the positive potential recorded from the superior cervical ganglia of several species. J Pharmacol Exp Ther. 1980 Nov;215(2):455–460. [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Shinnick-Gallagher P., Cole A. E., Griffith W. H., 3rd, Williams B. J. Current hypotheses for the slow inhibitory postsynaptic potential in sympathetic ganglia. Fed Proc. 1980 Oct;39(12):3009–3015. [PubMed] [Google Scholar]
- Hanbauer I., Johnson D. G., Silberstein S. D., Kopin I. J. Pharmacological and kinetic properties of uptake of ( 3 H)-norepinephrine by superior cervical ganglia of rats in organ culture. Neuropharmacology. 1972 Nov;11(6):857–862. doi: 10.1016/0028-3908(72)90044-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Ivanov A. Y., Skok V. I. Slow inhibitory postsynaptic potentials and hyperpolarization evoked by noradrenaline in the neurones of mammalian sympathetic ganglion. J Auton Nerv Syst. 1980 Mar;1(3):255–263. doi: 10.1016/0165-1838(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Adams P. R., Brownstein M. J., Rivier J. E. Teleost luteinizing hormone-releasing hormone: action on bullfrog sympathetic ganglia is consistent with role as neurotransmitter. J Neurosci. 1984 Feb;4(2):420–429. doi: 10.1523/JNEUROSCI.04-02-00420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Koketsu K., Nakamura M. The electrogenesis of adrenaline-hyperpolarization of sympathetic ganglion cells in bullfrogs. Jpn J Physiol. 1976;26(1):63–77. doi: 10.2170/jjphysiol.26.63. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Libet B., Kobayashi H. Adrenergic mediation of slow inhibitory postsynaptic potential in sympathetic ganglia of the frog. J Neurophysiol. 1974 Jul;37(4):805–814. doi: 10.1152/jn.1974.37.4.805. [DOI] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A. Examination of the role of the electrogenic sodium pump in the adrenaline-induced hyperpolarization of amphibian neurones. J Physiol. 1984 Feb;347:377–395. doi: 10.1113/jphysiol.1984.sp015071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Volle R. L. Responses of the rat superior cervical ganglion in vitro to isoprenaline and bethanechol. Naunyn Schmiedebergs Arch Pharmacol. 1978 Aug;304(1):15–20. doi: 10.1007/BF00501372. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Schulman J. A., Smith P. A., Busis N. A. Long-lasting synaptic potentials and the modulation of synaptic transmission. Fed Proc. 1979 Jun;38(7):2084–2094. [PubMed] [Google Scholar]
- Weight F. F., Weitsen H. A. Identification of small intensely fluorescent (SIF) cells as chromaffin cells in bullfrog sympathetic ganglia. Brain Res. 1977 Jun 10;128(2):213–226. doi: 10.1016/0006-8993(77)90989-1. [DOI] [PubMed] [Google Scholar]


