Abstract
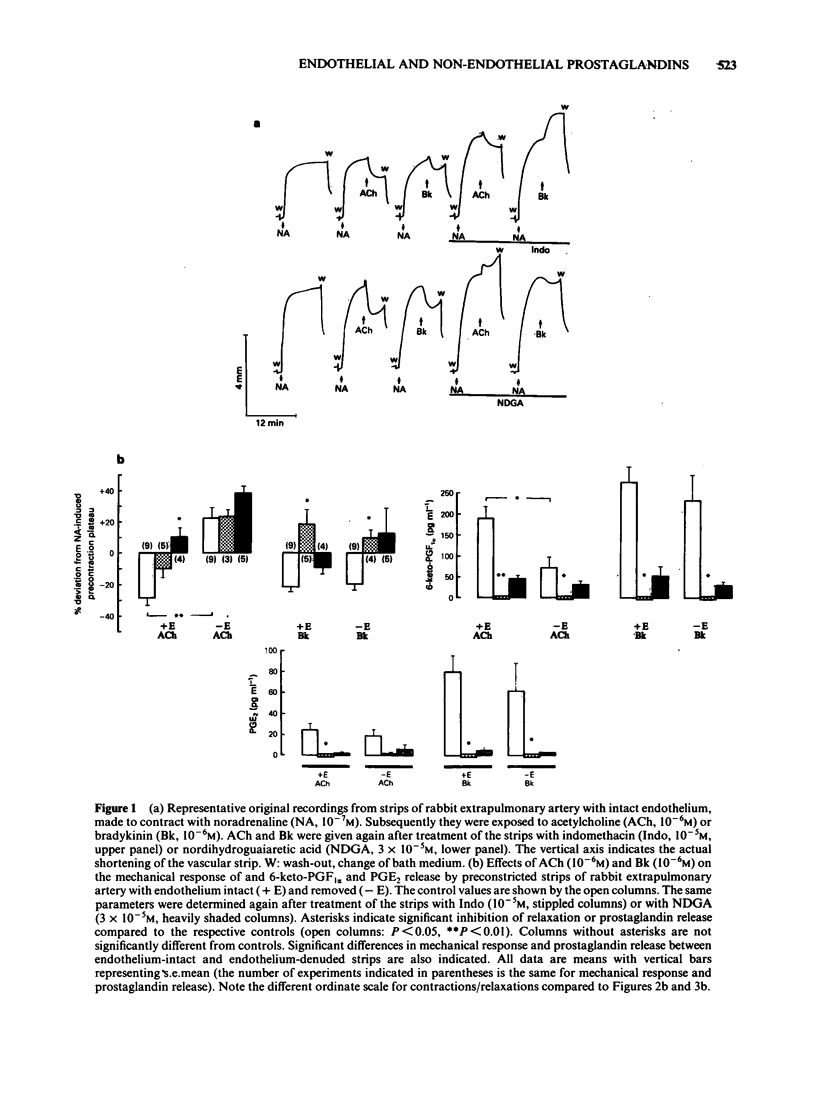
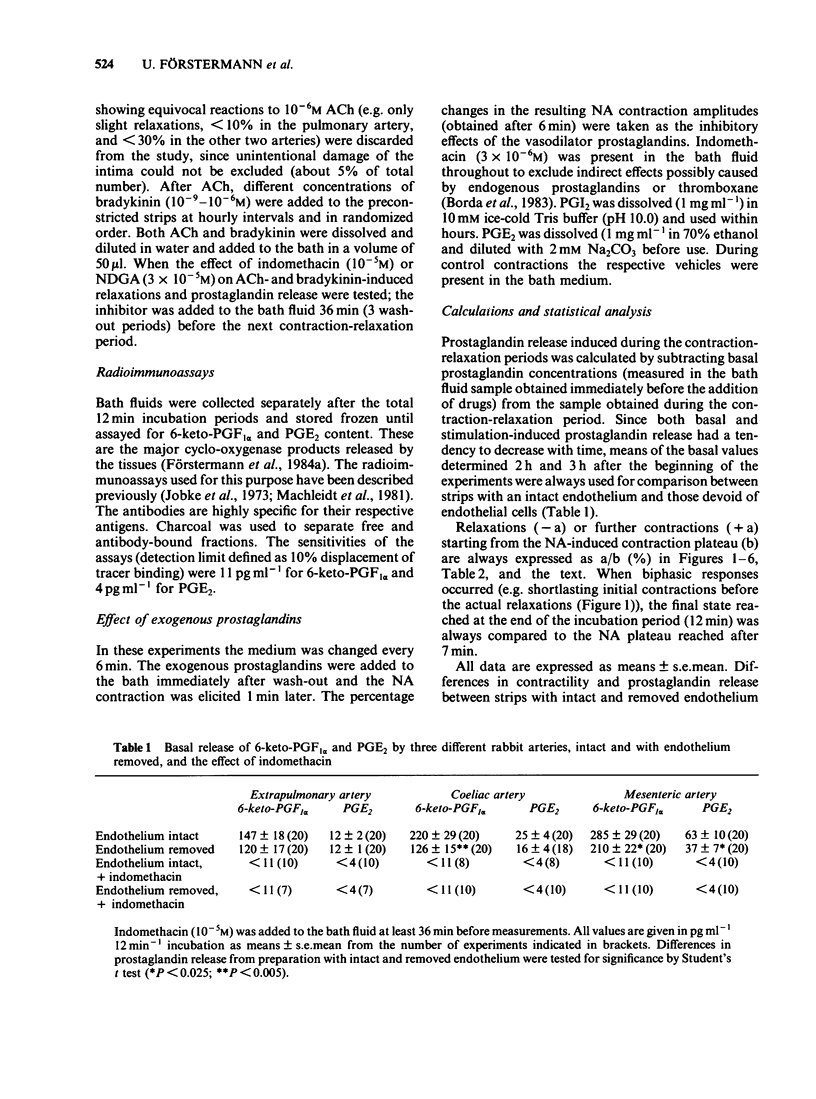
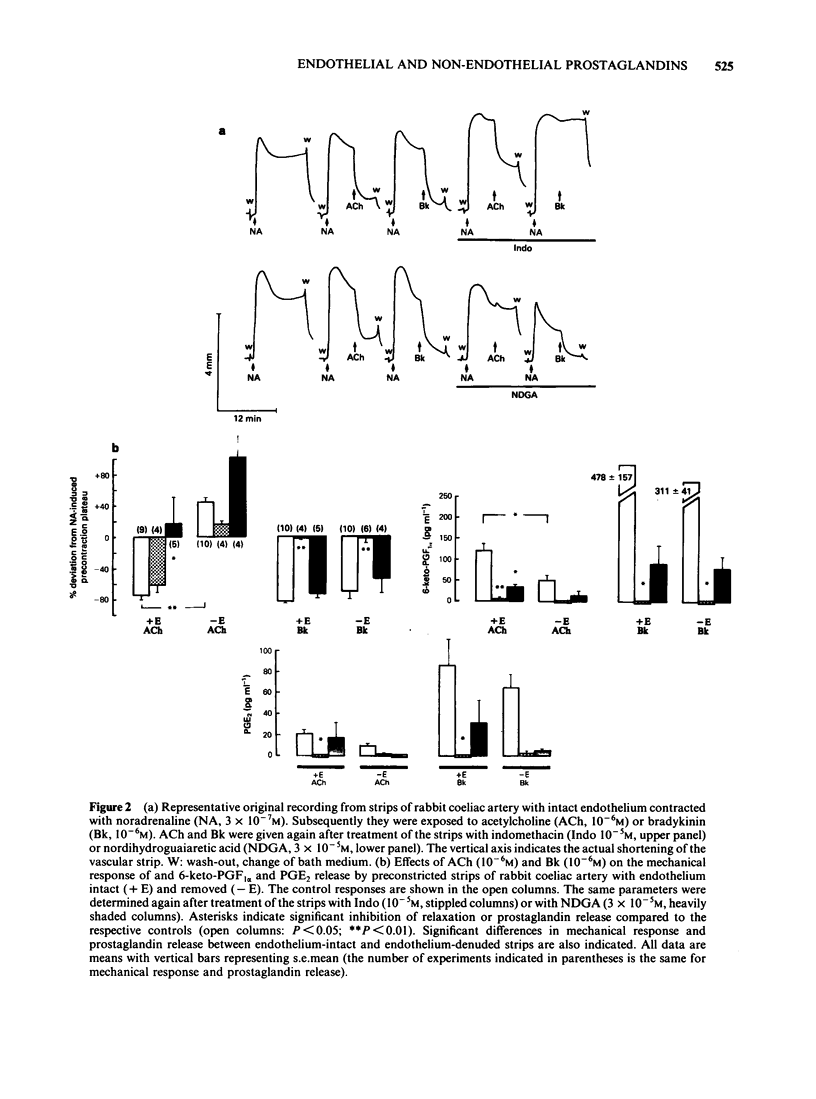
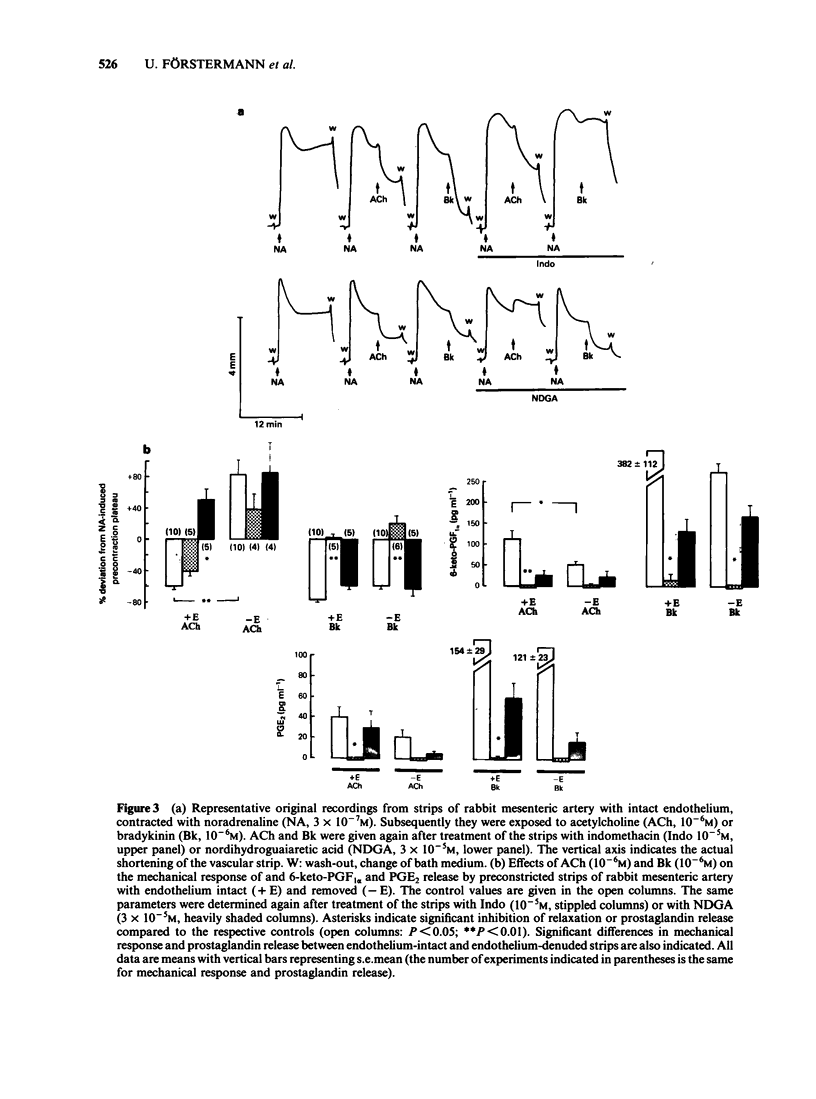
Strips of rabbit extrapulmonary, coeliac and mesenteric arteries were mounted in organ baths for isotonic recording of changes in tissue length. The formation by the strips of the vasodilator prostaglandins PGI2 (measured as 6-keto-PGF1 alpha) and PGE2 was determined by specific radioimmunoassays. Removal of vascular endothelium initially increased and then permanently decreased the basal prostaglandin release of the tissues. Acetylcholine (ACh) relaxed strips of all three arteries if the endothelium was intact. ACh also stimulated the formation of PGI2 and PGE2 from all three tissues; about 60% of these prostaglandins originated from endothelial cells. Indomethacin caused complete inhibition of prostaglandins formation and a slight inhibition of the ACh-relaxation (not statistically significant). Complete inhibition of the ACh relaxation was achieved with nordihydroguaiaretic acid (NDGA). NDGA also partially inhibited prostaglandin formation. These data suggest that in blood vessels that are also prostaglandin-sensitive, the ACh relaxation is predominantly mediated by a non-prostaglandin endothelium-derived relaxing factor. Bradykinin was more potent that ACh in releasing prostaglandins from the same arteries. This release was activated in subendothelial components of the vascular wall. Neither this prostaglandin release nor the bradykinin-induced relaxations were significantly reduced in endothelium-denuded arteries. Indomethacin completely blocked the bradykinin-induced prostaglandin release and the bradykinin relaxation. NDGA caused a moderate inhibition of the bradykinin-induced prostaglandin release and slightly attenuated the bradykinin relaxation (neither effect of NDGA was statistically significant). Under all experimental conditions (control, indomethacin, NDGA) and with all three arteries there was a good correlation between the bradykinin-induced prostaglandin release and the respective mechanical response. No such correlation could be found for ACh. Prostaglandin-dependent relaxations of the coeliac and mesenteric artery are probably mediated by endogenous PGI2. The extrapulmonary artery is rather insensitive to PGI2 and is probably relaxed mainly by endogenous PGE2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Gimbrone M. A., Jr Stimulation of prostaglandin E synthesis in cultured human umbilical vein smooth muscle cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1617–1620. doi: 10.1073/pnas.73.5.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura B. M., Chand N. Bradykinin-induced relaxation of renal and pulmonary arteries is dependent upon intact endothelial cells. Br J Pharmacol. 1981 Sep;74(1):10–11. doi: 10.1111/j.1476-5381.1981.tb09948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Beetens J. R., Van Hove C., Rampart M., Herman A. G. Acetylcholine stimulates the release of prostacyclin by rabbit aorta endothelium. J Pharm Pharmacol. 1983 Apr;35(4):251–252. doi: 10.1111/j.2042-7158.1983.tb02924.x. [DOI] [PubMed] [Google Scholar]
- Borda E. S., Sterin-Borda L., Gimeno M. F., Lazzari M. A., Gimeno A. L. The stimulatory effect of prostacyclin (PGI2) on isolated rabbit and rat aorta is probably associated to the generation of a thromboxane A2 (TXA2) "like-material". Arch Int Pharmacodyn Ther. 1983 Jan;261(1):79–89. [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Busse R., Förstermann U., Matsuda H., Pohl U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflugers Arch. 1984 May;401(1):77–83. doi: 10.1007/BF00581536. [DOI] [PubMed] [Google Scholar]
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Hertting G., Neufang B. The importance of endogenous prostaglandins other than prostacyclin, for the modulation of contractility of some rabbit blood vessels. Br J Pharmacol. 1984 Apr;81(4):623–630. doi: 10.1111/j.1476-5381.1984.tb16127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. Endothelium-dependent vasodilation by melittin: are lipoxygenase products involved? Am J Physiol. 1985 Jul;249(1 Pt 2):H14–H19. doi: 10.1152/ajpheart.1985.249.1.H14. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Trogisch G., Busse R. Species-dependent differences in the nature of endothelium-derived vascular relaxing factor. Eur J Pharmacol. 1984 Nov 27;106(3):639–643. doi: 10.1016/0014-2999(84)90071-2. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. C. Contribution of the subendothelium to prostacyclin release after vascular injury. J Lab Clin Med. 1982 Oct;100(4):574–584. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Herman A. G., Moncada S., Vane J. R. Formation of prostacyclin (PGl2) by different layers of the arterial wall. Arch Int Pharmacodyn Ther. 1977 May;227(1):162–163. [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Silver M. J., Smith J. B., Nissenbaum M., Sedar A. W. Prostacyclin production in rabbit arteries in situ: inhibition by arachidonic acid-induced endothelial cell damage or by low-dose aspirin. Prostaglandins. 1981 Apr;21(4):655–666. doi: 10.1016/0090-6980(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Jobke A., Peskar B. A., Peskar B. M. On the specificity of antisera against prostaglandins A2 and E2. FEBS Lett. 1973 Dec 1;37(2):192–196. doi: 10.1016/0014-5793(73)80456-9. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Pearson J. D., Gordon J. L. Localisation and stimulation of prostacyclin production in vascular cells. Nature. 1978 Feb 9;271(5645):549–551. doi: 10.1038/271549a0. [DOI] [PubMed] [Google Scholar]
- Machleidt C., Förstermann U., Anhut H., Hertting G. Formation and elimination of prostacyclin metabolites in the cat in vivo as determined by radioimmunoassay of unextracted plasma. Eur J Pharmacol. 1981 Aug 27;74(1):19–26. doi: 10.1016/0014-2999(81)90318-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Endothelium-dependent relaxation of rabbit aorta. II. Inhibition of relaxation stimulated by methacholine and A23187 with antagonists of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983 Sep;226(3):796–801. [PubMed] [Google Scholar]
- Van de Voorde J., Leusen I. Role of the endothelium in the vasodilator response of rat thoracic aorta to histamine. Eur J Pharmacol. 1983 Jan 28;87(1):113–120. doi: 10.1016/0014-2999(83)90056-0. [DOI] [PubMed] [Google Scholar]


