Abstract
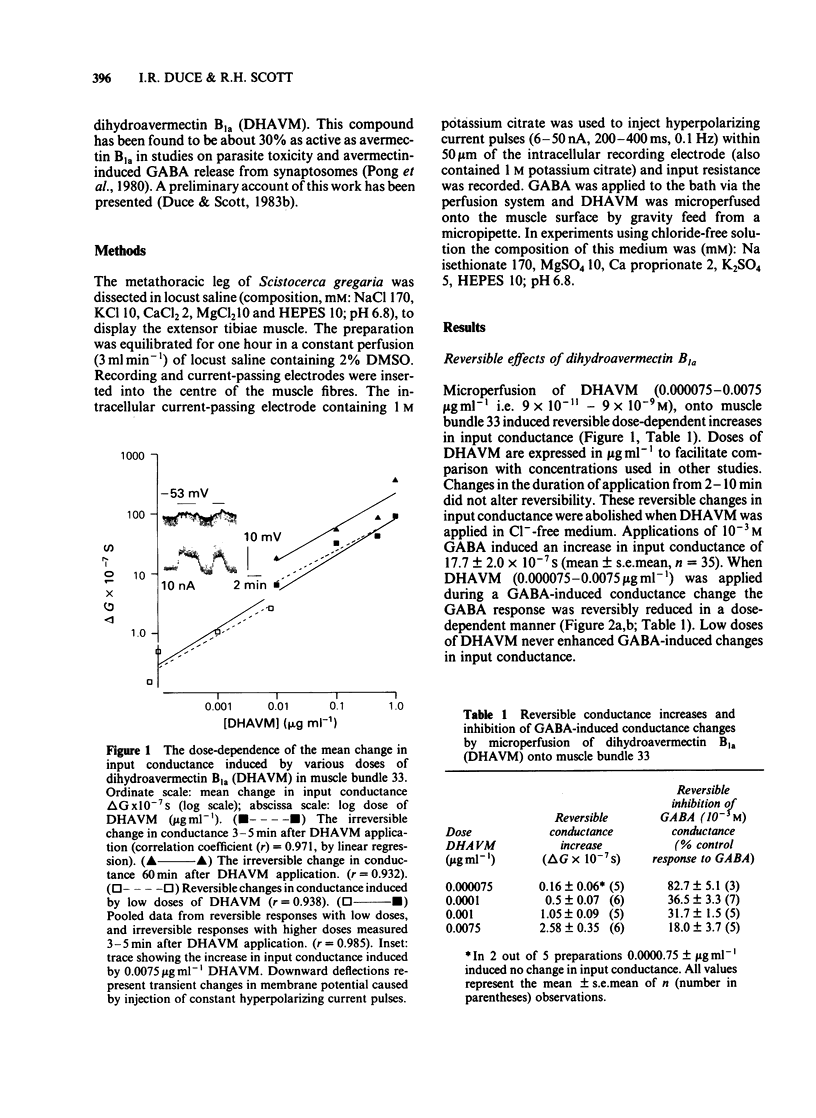
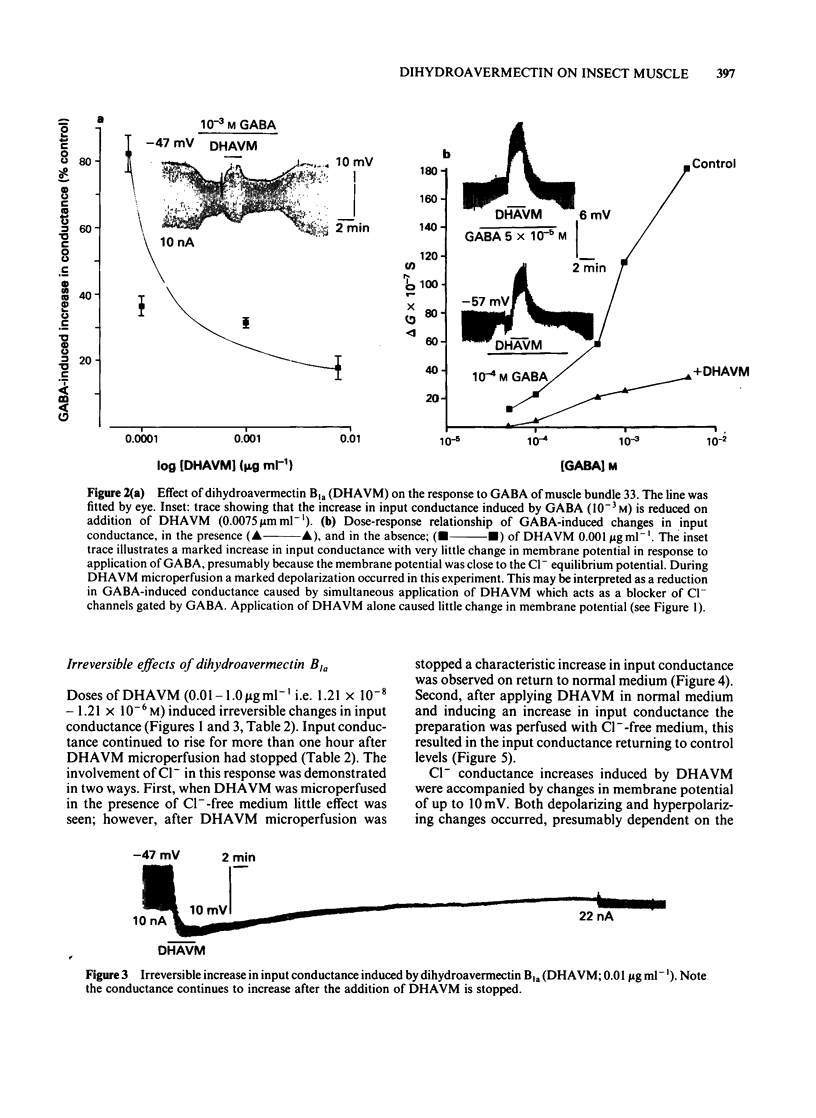
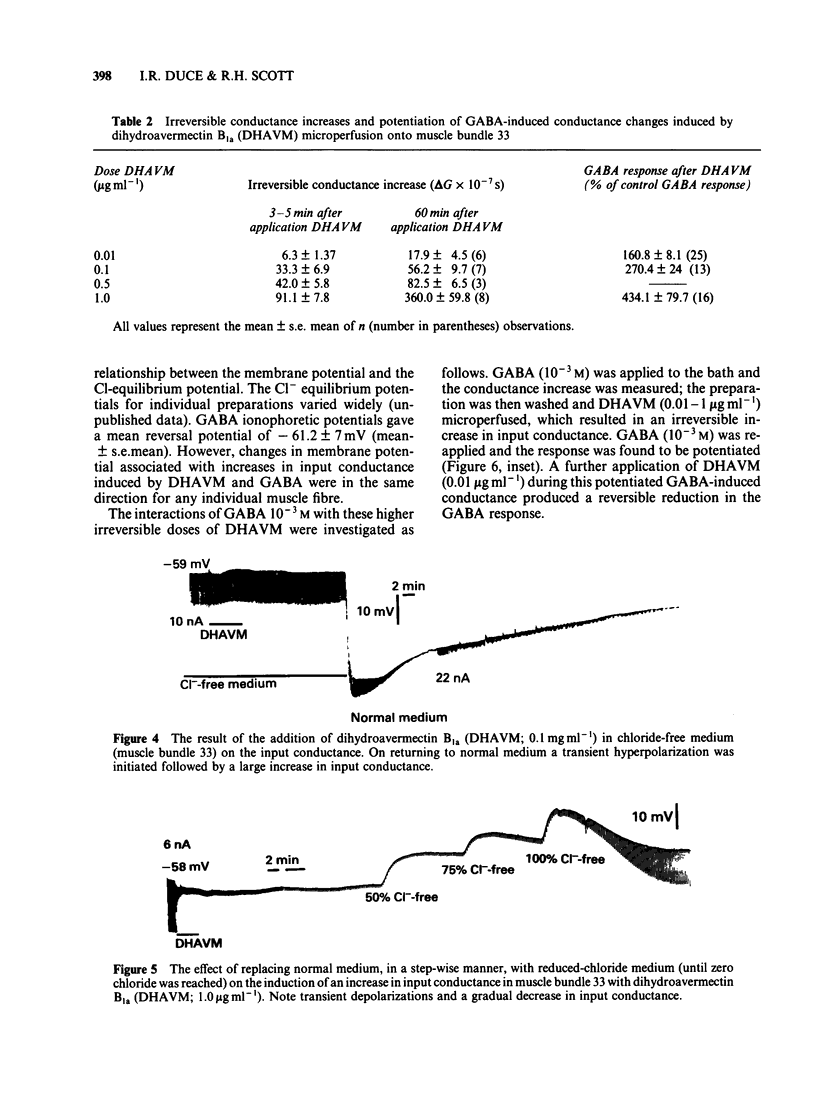
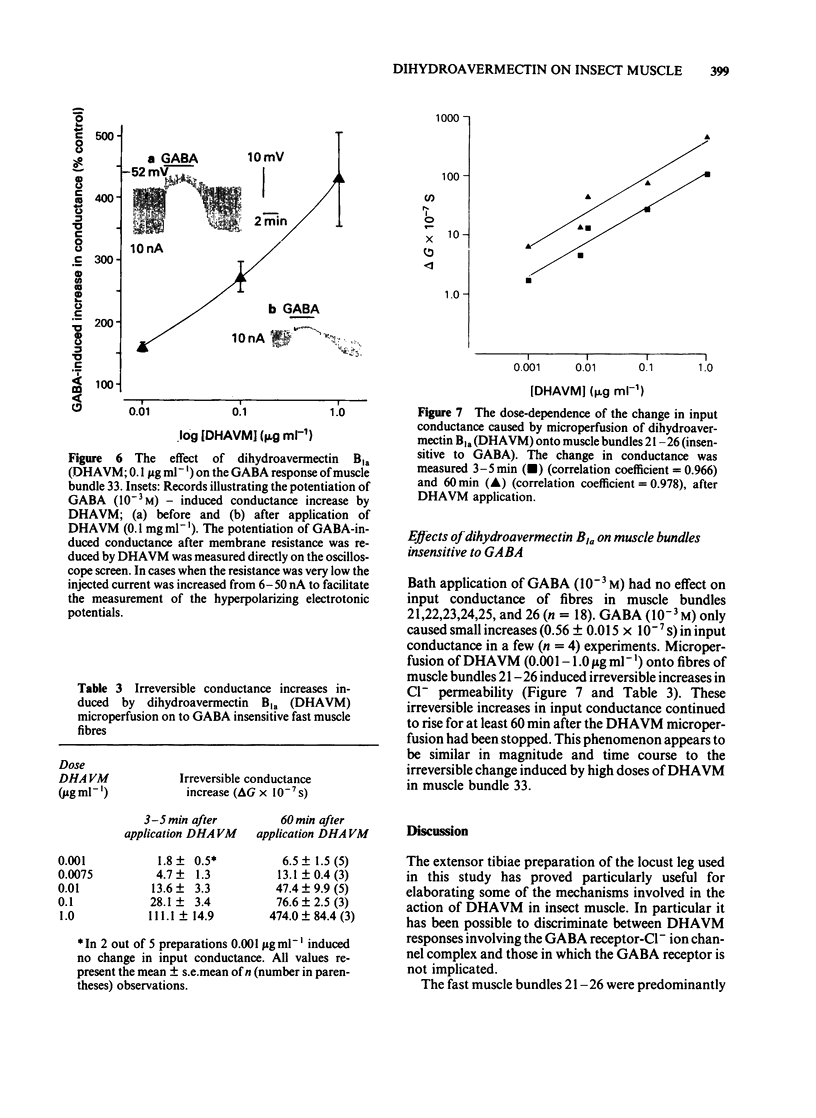
Muscle bundle 33 of the locust (Schistocerca gregaria) extensor tibiae muscle which is sensitive to gamma-aminobutyric acid (GABA) and receives inhibitory innervation, exhibited both reversible and irreversible responses to dihydroavermectin B1a (DHAVM). These responses involved increases in C1- permeability. DHAVM (0.000075-0.0075 microgram ml-1) induced reversible dose-dependent increases in C1- permeability and partially blocked GABA-induced C1- conductance. These effects appear to be due to an interaction of DHAVM with the GABA receptor-C1- ion channel complex. DHAVM (0.01-1.0 microgram ml-1) induced an irreversible increase in C1- conductance which continued to rise after DHAVM application was stopped. At these concentrations DHAVM potentiated GABA-induced C1- conductances which were in turn reduced by microperfusion of DHAVM (0.01-1.0 microgram ml-1) during bath application of GABA. DHAVM (0.0001-1.0 microgram ml-1) induced only irreversible increases in C1- conductance when applied to fast muscle bundles (21-26) of the locust extensor tibiae muscle, which are GABA-insensitive and have no inhibitory innervation. The actions of DHAVM on locust muscle appear to involve more than one site. Reversible actions of DHAVM appear to be related to GABA sensitivity and may involve the GABA receptor-ionophore complex. This is unlikely to be the site of action for the irreversible increases in C1- conductance caused by DHAVM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg R. W., Miller B. M., Baker E. E., Birnbaum J., Currie S. A., Hartman R., Kong Y. L., Monaghan R. L., Olson G., Putter I. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979 Mar;15(3):361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R. Junctional and extrajunctional membrane channels activated by GABA in locust muscle fibres. Proc R Soc Lond B Biol Sci. 1981 Mar 27;211(1185):527–535. doi: 10.1098/rspb.1981.0021. [DOI] [PubMed] [Google Scholar]
- Egerton J. R., Ostlind D. A., Blair L. S., Eary C. H., Suhayda D., Cifelli S., Riek R. F., Campbell W. C. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 1979 Mar;15(3):372–378. doi: 10.1128/aac.15.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Wang C. C., Gorio A. Avermectin B1a irreversibly blocks postsynaptic potentials at the lobster neuromuscular junction by reducing muscle membrane resistance. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2062–2066. doi: 10.1073/pnas.76.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P. Effects of anesthetics on ion channels in synapses. Int Rev Physiol. 1981;25:1–45. [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Kass I. S., Wang C. C., Walrond J. P., Stretton A. O. Avermectin B1a, a paralyzing anthelmintic that affects interneurons and inhibitory motoneurons in Ascaris. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6211–6215. doi: 10.1073/pnas.77.10.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin T. N., Busch R. D., Wang C. C. Postsynaptic inhibition of invertebrate neuromuscular transmission by avermectin B1a. Neuropharmacology. 1983 Jan;22(1):89–96. doi: 10.1016/0028-3908(83)90265-4. [DOI] [PubMed] [Google Scholar]
- Ostlind D. A., Cifelli S., Lang R. Insecticidal activity of the anti-parasitic avermectins. Vet Rec. 1979 Aug 25;105(8):168–168. doi: 10.1136/vr.105.8.168-a. [DOI] [PubMed] [Google Scholar]
- Pong S. S., Wang C. C. Avermectin B1a modulation of gamma-aminobutyric acid receptors in rat brain membranes. J Neurochem. 1982 Feb;38(2):375–379. doi: 10.1111/j.1471-4159.1982.tb08639.x. [DOI] [PubMed] [Google Scholar]
- Pong S. S., Wang C. C., Fritz L. C. Studies on the mechanism of action of avermectin B1a: stimulation of release of gamma-aminobutyric acid from brain synaptosomes. J Neurochem. 1980 Feb;34(2):351–358. doi: 10.1111/j.1471-4159.1980.tb06604.x. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Williams M., Yarbrough G. G. Enhancement of in vitro binding and some of the pharmacological properties of diazepam by a novel anthelmintic agent, Avermectin B1a. Eur J Pharmacol. 1979 Jun 15;56(3):273–276. doi: 10.1016/0014-2999(79)90183-3. [DOI] [PubMed] [Google Scholar]


