Abstract
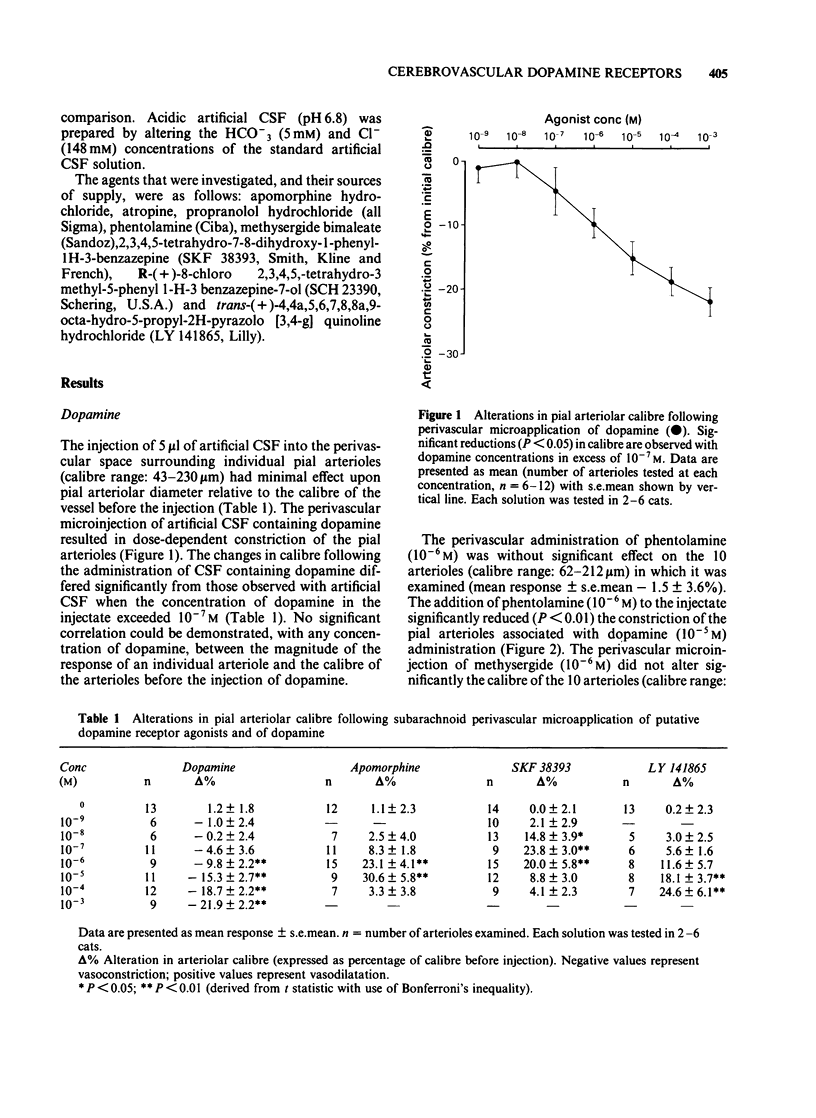
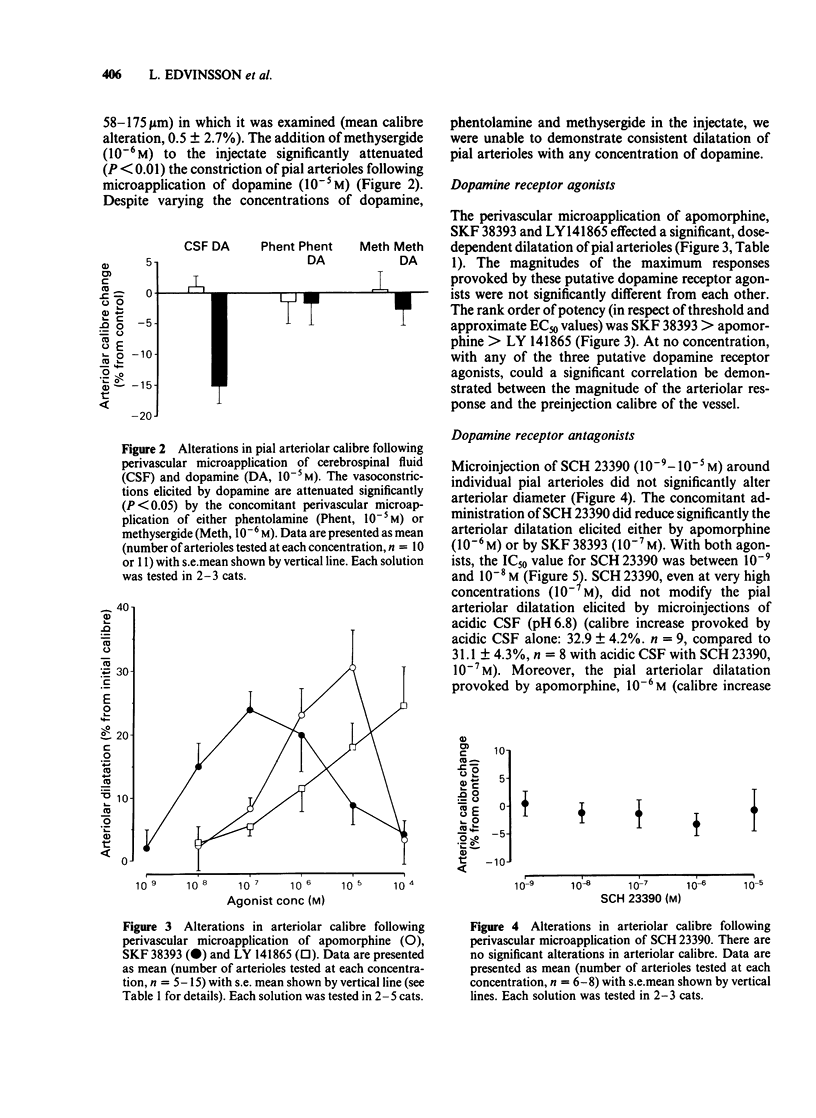
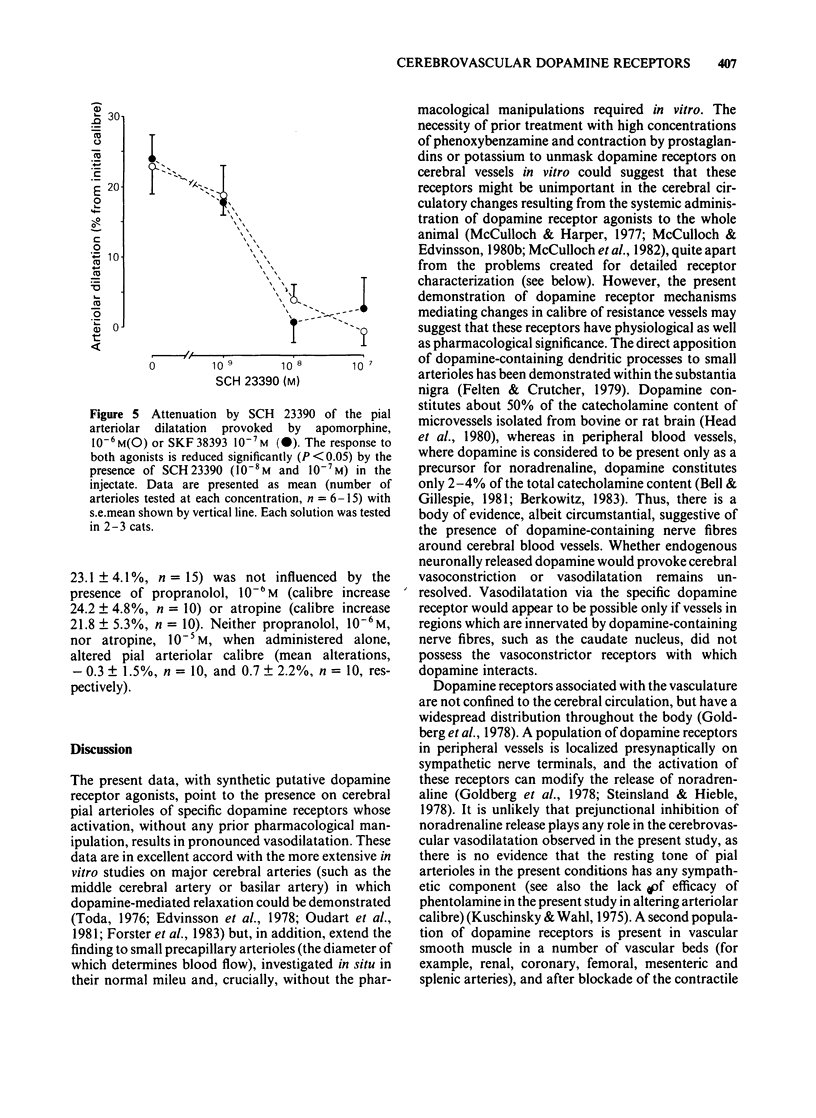
The vasomotor responses of individual cerebral pial arterioles on the convexity of the cerebral cortex to subarachnoid perivascular micro-injections of dopamine and the putative dopamine receptor agonists, apomorphine, SKF 38393 and LY 141865, have been examined in 38 anaesthetized cats. The perivascular microapplication of dopamine (10(-9)-10(-3)M) effected dose-dependent reductions in pial arteriolar calibre, with the maximum reductions in calibre (22 +/- 2% from preinjection levels: mean +/- s.e.) being observed at 10(-3)M. The cerebrovascular constriction produced by dopamine (10(-5)M) could be significantly attenuated by the concomitant perivascular administration of phentolamine (10(-6)M) or methysergide (10(-6)M). The perivascular microapplication of apomorphine (10(-8)-10(-4)M) effected dose-dependent increases in arteriolar calibre, with the maximum increase (31 +/- 6%) being observed with apomorphine (10(-5)M). The perivascular administration of the putative dopamine D1-receptor agonist, SKF 38393 (10(-9)-10(-4)M) increased arteriolar calibre, with the maximum response (24 +/- 3%) being observed with injection of 10(-7)M. The putative dopamine D2-receptor agonist, LY 141865, also increased cerebral arteriolar calibre, but only at high concentrations (maximum calibre increase 25 +/- 6.1 with 10(-4)M). The cerebrovascular dilatations elicited by apomorphine and by SKF 38393 were markedly attenuated by the concomitant perivascular microapplication of the putative dopamine D1-receptor antagonist, SCH 23390 (10(-8)M). The perivascular administration of SCH 23390 (10(-9)-10(-5)M) per se did not alter arteriolar calibre nor the arteriolar dilatation provoked by microinjections of acidic cerebrospinal fluid. These results point to the presence on cat cerebral arterioles of dopamine receptors (probably of D1 subtype) mediating dilation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Gebrewold A., Lassoff S. Biphasic responsiveness of rat pial arterioles to dopamine: direct observations on the microcirculation. Br J Pharmacol. 1980 Aug;69(4):543–544. doi: 10.1111/j.1476-5381.1980.tb07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F., Cavallotti C., De Rossi M., Mione M. C. Dopamine-sensitive cAMP generating system in rat extracerebral arteries. Eur J Pharmacol. 1984 Jan 13;97(1-2):105–109. doi: 10.1016/0014-2999(84)90517-x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. M., Duval N., Langer S. Z. LY 141865 stimulates histamine-2 (H2) receptors and dopamine-2 (DA2) receptors in the anaesthetised dog. Eur J Pharmacol. 1983 Jan 28;87(1):165–166. doi: 10.1016/0014-2999(83)90067-5. [DOI] [PubMed] [Google Scholar]
- Baca G. M., Palmer G. C. Presence of hormonally-sensitive adenylate cyclase receptors in capillary-enriched fractions from rat cerebral cortex. Blood Vessels. 1978;15(5):286–298. doi: 10.1159/000158174. [DOI] [PubMed] [Google Scholar]
- Bell C., Gillespie J. S. Dopamine and noradrenaline levels in peripheral tissues of several mammalian species. J Neurochem. 1981 Feb;36(2):703–706. doi: 10.1111/j.1471-4159.1981.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz B. A. Dopamine and dopamine receptors as target sites for cardiovascular drug action. Fed Proc. 1983 Oct;42(13):3019–3021. [PubMed] [Google Scholar]
- Cross A. J., Mashal R. D., Johnson J. A., Owen F. Preferential inhibition of ligand binding to calf striatal dopamine D1 receptors by SCH 23390. Neuropharmacology. 1983 Nov;22(11):1327–1329. doi: 10.1016/0028-3908(83)90208-3. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Hardebo J. E., McCulloch J., Owman C. Effects of dopaminergic agonists and antagonists on isolated cerebral blood vessels. Acta Physiol Scand. 1978 Nov;104(3):349–359. doi: 10.1111/j.1748-1716.1978.tb06286.x. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Crutcher K. A. Neuronal-vascular relationships in the raphe nuclei, locus coeruleus, and substantia nigra in primates. Am J Anat. 1979 Aug;155(4):467–481. doi: 10.1002/aja.1001550405. [DOI] [PubMed] [Google Scholar]
- Forster C., Drew G. M., Hilditch A., Whalley E. T. Dopamine receptors in human basilar arteries. Eur J Pharmacol. 1983 Feb 18;87(2-3):227–235. doi: 10.1016/0014-2999(83)90332-1. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I., Toda N. Dopamine induced relaxation of isolated canine renal, mesenteric, and femoral arteries contracted with prostaglandin F2-alpha. Circ Res. 1975 Jun;36(6 Suppl 1):97–102. doi: 10.1161/01.res.36.6.97. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I., Volkman P. H., Kohli J. D. A comparison of the vascular dopamine receptor with other dopamine receptors. Annu Rev Pharmacol Toxicol. 1978;18:57–79. doi: 10.1146/annurev.pa.18.040178.000421. [DOI] [PubMed] [Google Scholar]
- Hamblin M. W., Creese I. Phenoxybenzamine treatment differentiates dopaminergic 3H-ligand binding sites in bovine caudate membranes. Mol Pharmacol. 1982 Jan;21(1):44–51. [PubMed] [Google Scholar]
- Head R. J., Hjelle J. T., Jarrott B., Berkowitz B., Cardinale G., Spector S. Isolated brain microvessels: preparation, morphology, histamine and catecholamine contents. Blood Vessels. 1980;17(4):173–186. doi: 10.1159/000158247. [DOI] [PubMed] [Google Scholar]
- Hilditch A., Drew G. M. Characteristics of the dopamine receptors in the rabbit isolate splenic artery. Eur J Pharmacol. 1981 Jul 10;72(4):287–296. doi: 10.1016/0014-2999(81)90566-5. [DOI] [PubMed] [Google Scholar]
- Hilditch A., Drew G. M., Naylor R. J. SCH 23390 is a very potent and selective antagonist at vascular dopamine receptors. Eur J Pharmacol. 1984 Jan 27;97(3-4):333–334. doi: 10.1016/0014-2999(84)90471-0. [DOI] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Leitz F. H., Houser V. P., Korduba C. A. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983 Aug;226(2):462–468. [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M. Alpha-receptor stimulation by endogenous and exogenous norepinephrine and blockade by phentolamine in pial arteries of cats. Circ Res. 1975 Aug;37(2):168–174. doi: 10.1161/01.res.37.2.168. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Edvinsson L. Cerebral circulatory and metabolic effects of piribedil. Eur J Pharmacol. 1980 Sep 5;66(4):327–337. doi: 10.1016/0014-2999(80)90465-3. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Edvinsson L. Cerebral circulatory and metabolic effects of vasoactive intestinal polypeptide. Am J Physiol. 1980 Apr;238(4):H449–H456. doi: 10.1152/ajpheart.1980.238.4.H449. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Edvinsson L. Cerebrovascular smooth muscle reactivity: a critical appraisal of in vitro and in situ techniques. J Cereb Blood Flow Metab. 1984 Jun;4(2):129–139. doi: 10.1038/jcbfm.1984.21. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Harper A. M. Cerebral circulation: effect of stimulation and blockade of dopamine receptors. Am J Physiol. 1977 Aug;233(2):H222–H227. doi: 10.1152/ajpheart.1977.233.2.H222. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Kelly P. A., Ford I. Effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow (with an appendix on its statistical analysis). J Cereb Blood Flow Metab. 1982 Dec;2(4):487–499. doi: 10.1038/jcbfm.1982.56. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Savaki H. E., McCulloch M. C., Sokoloff L. Specific distribution of metabolic alterations in cerebral cortex following apomorphine administration. Nature. 1979 Nov 15;282(5736):303–305. doi: 10.1038/282303a0. [DOI] [PubMed] [Google Scholar]
- O'Boyle K. M., Waddington J. L. Selective and stereospecific interactions of R-SK & F 38393 with [3H]piflutixol but not [3H]spiperone binding to striatal D1 and D2 dopamine receptors: comparisons with SCH 23390. Eur J Pharmacol. 1984 Mar 2;98(3-4):433–436. doi: 10.1016/0014-2999(84)90294-2. [DOI] [PubMed] [Google Scholar]
- Oudart N., Sercombe R., Aubineau P., Boulu R. G., Seylaz J. Relaxation by dopaminergic agonists in cerebral and peripheral arteries (in vitro). Arch Int Pharmacodyn Ther. 1981 Aug;252(2):196–209. [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Shaar C. J. Relative potency of LY141865 at dopamine DA2 and histamine H2 receptors. Eur J Pharmacol. 1983 Sep 2;92(3-4):295–296. doi: 10.1016/0014-2999(83)90302-3. [DOI] [PubMed] [Google Scholar]
- Scatton B. Further evidence for the involvement of D2, but not D1 dopamine receptors in dopaminergic control of striatal cholinergic transmission. Life Sci. 1982 Dec 20;31(25):2883–2890. doi: 10.1016/0024-3205(82)90679-8. [DOI] [PubMed] [Google Scholar]
- Setler P. E., Sarau H. M., Zirkle C. L., Saunders H. L. The central effects of a novel dopamine agonist. Eur J Pharmacol. 1978 Aug 15;50(4):419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Steinsland O. S., Hieble J. P. Dopaminergic inhibition of adrenergic neurotransmission as a model for studies on dopamine receptor mechanisms. Science. 1978 Jan 27;199(4327):443–445. doi: 10.1126/science.22933. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Independent in vitro regulation by the D-2 dopamine receptor of dopamine-stimulated efflux of cyclic AMP and K+-stimulated release of acetylcholine from rat neostriatum. Brain Res. 1982 Nov 4;250(2):263–270. doi: 10.1016/0006-8993(82)90420-6. [DOI] [PubMed] [Google Scholar]
- Toda N. Heterogeneity in the response to dopamine of monkey cerebral and peripheral arteries. Am J Physiol. 1983 Dec;245(6):H930–H936. doi: 10.1152/ajpheart.1983.245.6.H930. [DOI] [PubMed] [Google Scholar]
- Toda N. Influence of dopamine and noradrenaline on isolated cerebral arteries of the dog. Br J Pharmacol. 1976 Sep;58(1):121–126. doi: 10.1111/j.1476-5381.1976.tb07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta K., Frey E. A., Grewe C. W., Cote T. E., Eskay R. L., Kebabian J. W. Evidence that LY-141865 specifically stimulates the D-2 dopamine receptor. Nature. 1981 Jul 30;292(5822):463–465. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Walton K. G., Liepmann P., Baldessarini R. J. Inhibition of dopamine-stimulated adenylate cyclase activity by phenoxybenzamine. Eur J Pharmacol. 1978 Nov 15;52(2):231–234. doi: 10.1016/0014-2999(78)90211-x. [DOI] [PubMed] [Google Scholar]


