Abstract
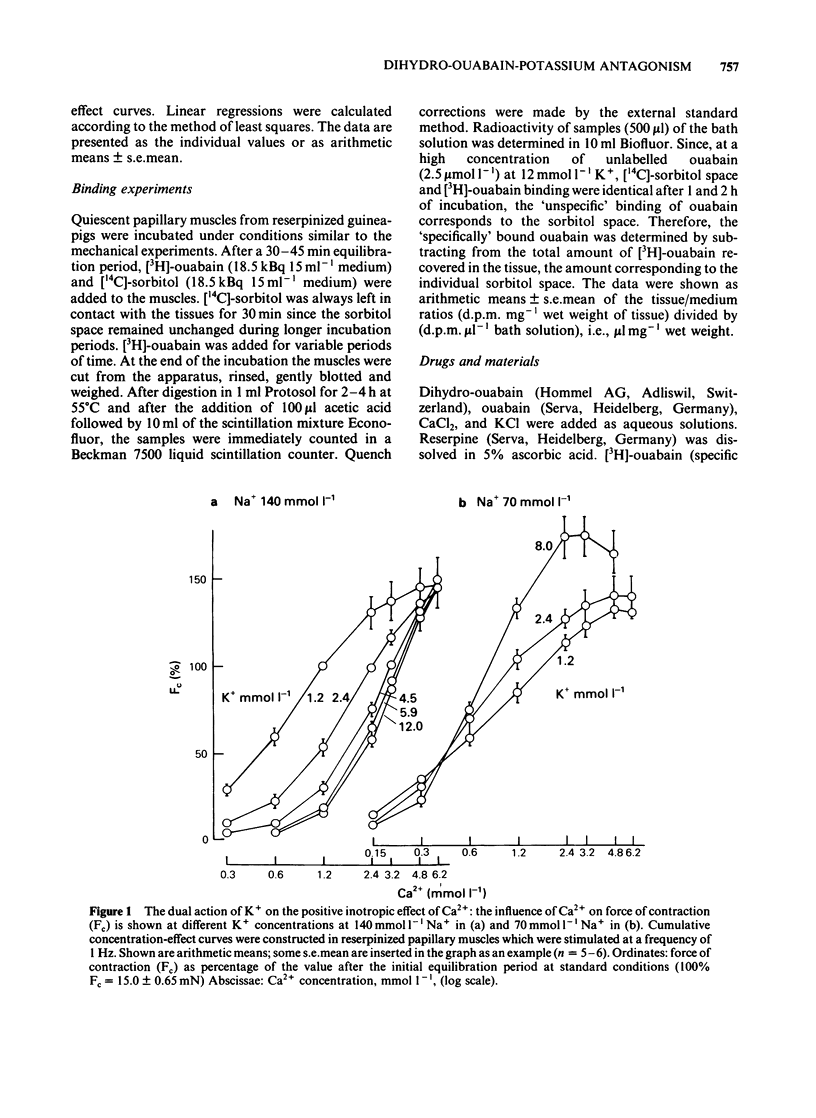
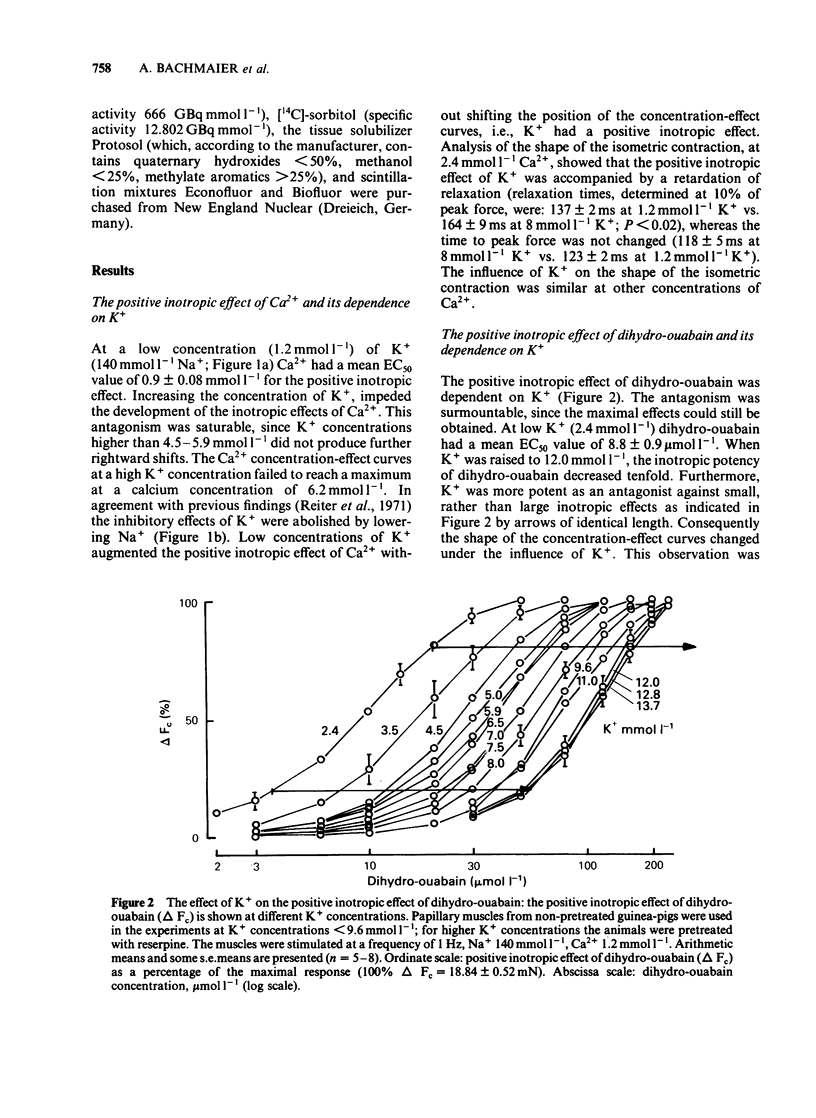
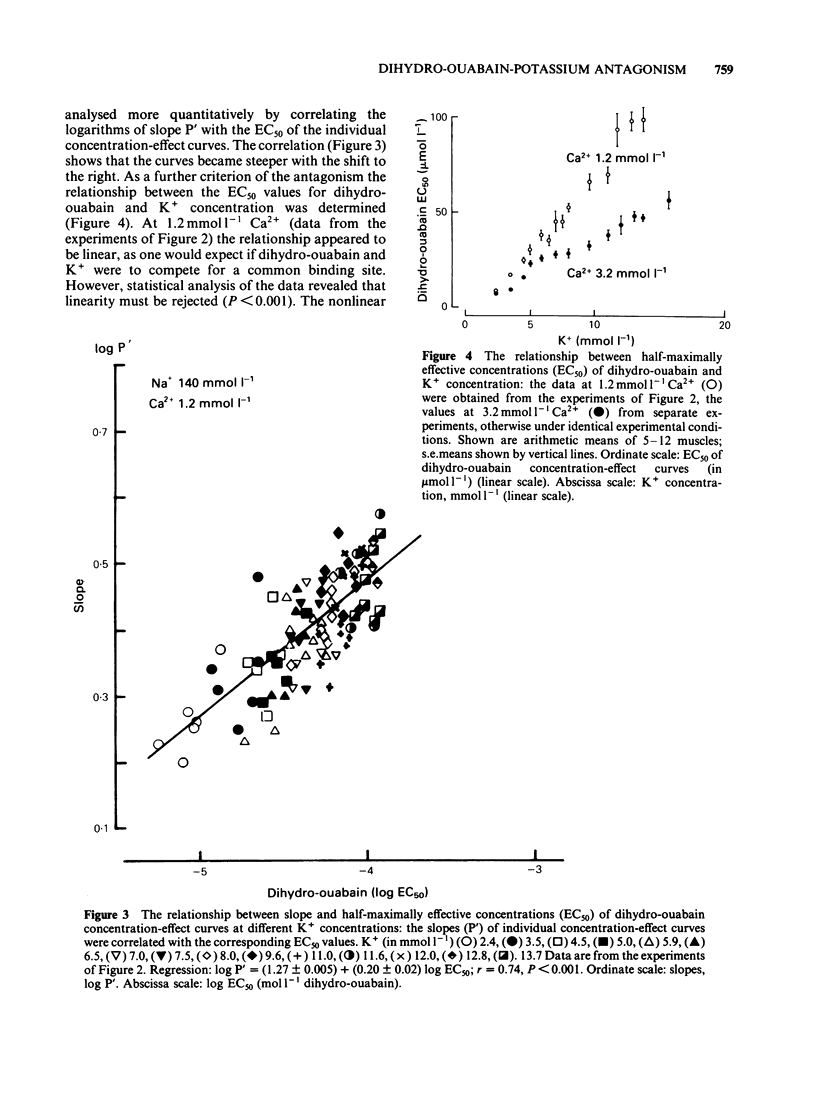
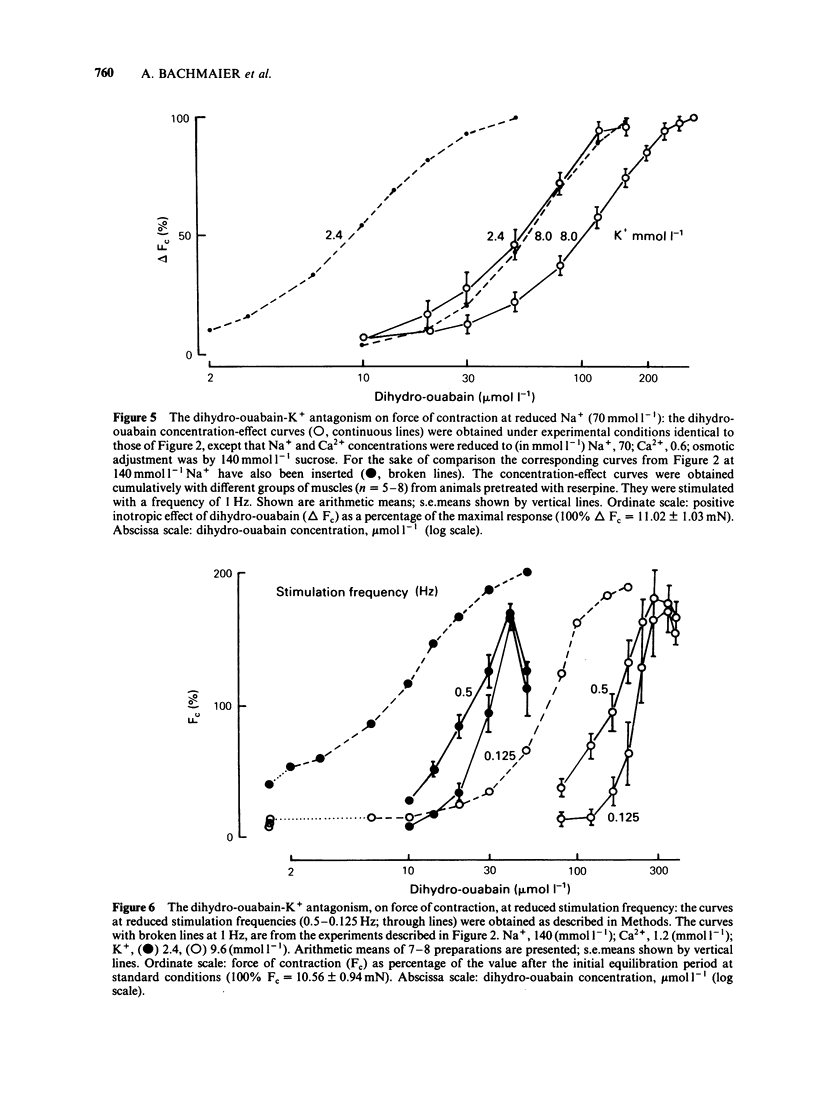
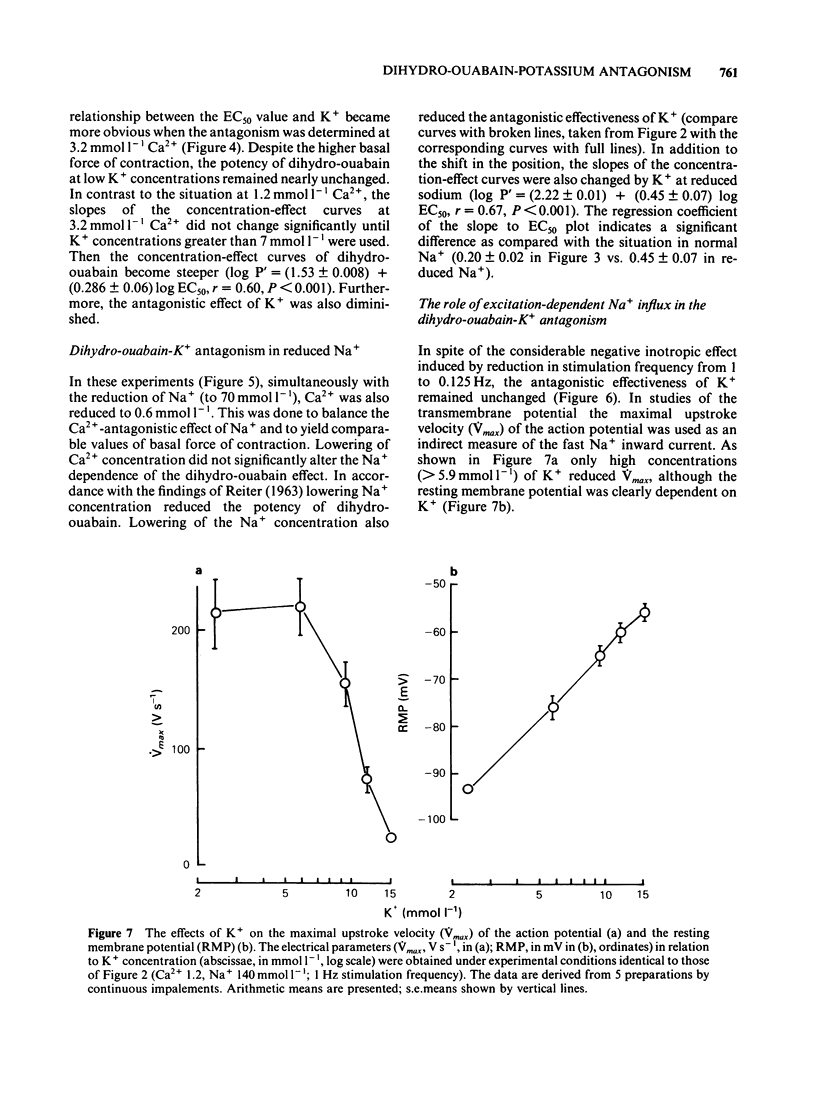
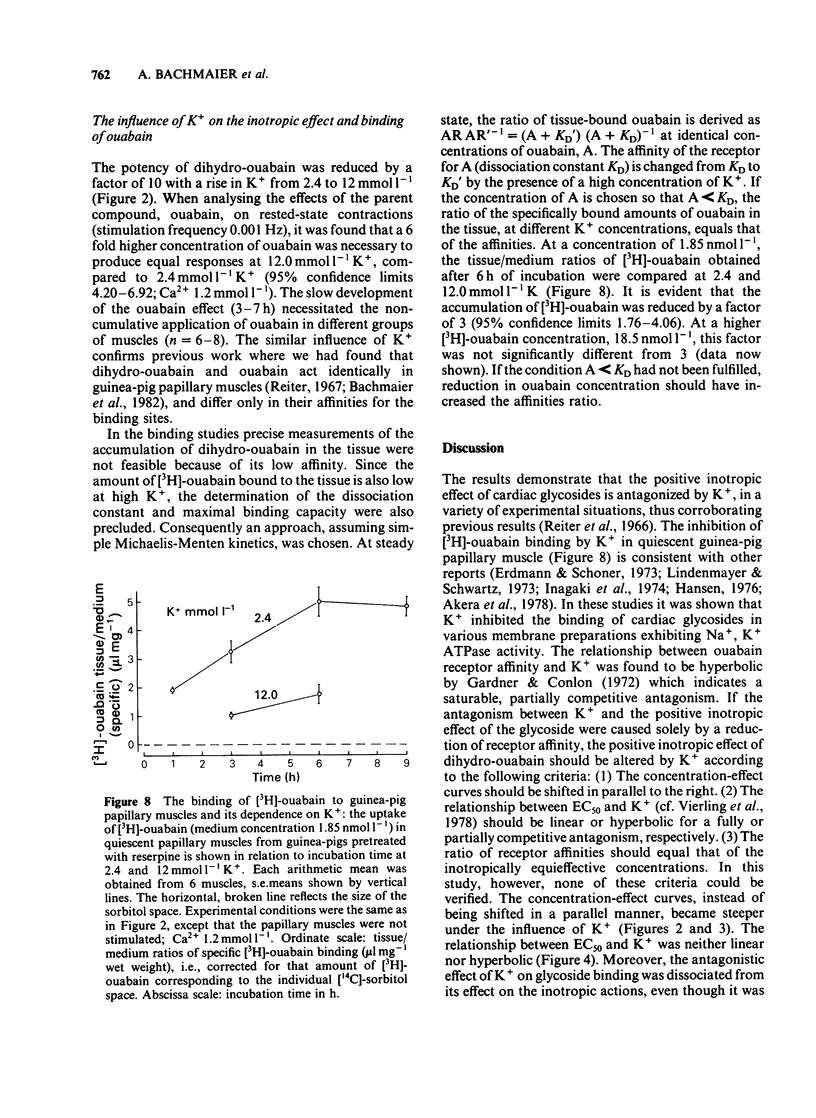
K+ (2.4-15.6 mmol l-1) antagonized the positive inotropic effect of dihydro-ouabain. The concentration-effect curves became steeper with the shift to higher concentrations of the glycoside. At 1.2 mmol l-1 Ca2+, an increase in K+ from 2.4 to 12 mmol l-1 required tenfold higher concentrations of dihydro-ouabain to produce equal inotropic effects. This factor was reduced to four at 3.2 mmol l-1 Ca2+. The same change in K+ concentration, at 1.2 mmol l-1 Ca2+, diminished the inotropic effect of ouabain on rested-state contractions by a factor of six. The positive inotropic effect of Ca2+ was also antagonized by K+ (1.2-12 mmol l-1). Reduction of Na+ from 140 to 70 mmol l-1 abolished the antagonistic action of K+ (1.2-8.0 mmol l-1). Moreover the inotropic effect of Ca2+ was enhanced. Reduction of Na+, from 140 to 70 mmol l-1, antagonized the positive inotropic effect of dihydro-ouabain more at low (2.4 mmol l-1) than at high (8.0 mmol l-1) K+. Accordingly, the extent of the dihydro-ouabain-K+ antagonism was reduced. When the K+ concentration was increased from 2.4 to 12 mmol l-1, [3H]-ouabain binding was reduced by a factor of three. This is less than the reduction in the inotropic effectiveness of ouabain or dihydro-ouabain. Reduction of stimulation frequency from 1 to 0.1215 Hz did not significantly alter the antagonistic effect of K+. Diminution of Vmax of the action potential was observed only at K+ concentrations greater than 5.9 mmol l-1, whereas the resting membrane potential was continuously depolarized over the entire range of K+ concentrations. The results support the view that the reduction in receptor affinity cannot be the sole cause of the antagonism between the glycoside and K+. Impairment of passive Na+ influx during diastole, due to the K+-dependent depolarization of the resting membrane potential, contributed to about one half of the glycoside-K+ antagonism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Temma K., Wiest S. A., Brody T. M. Reduction of the equilibrium binding of cardiac glycosides and related compounds to Na+,K+-ATPase as a possible mechanism for the potassium-induced reversal of their toxicity. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):157–165. doi: 10.1007/BF00495552. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselen P. Effect of potassium depolarization on sodium-dependent calcium efflux from goldfish heart ventricles and guinea-pig atria. J Physiol. 1982 Jun;327:309–324. doi: 10.1113/jphysiol.1982.sp014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The interaction of sodium and calcium ions at the cell membrane and the control of contractile strength in frog atrial muscle. J Physiol. 1980 Aug;305:109–123. doi: 10.1113/jphysiol.1980.sp013353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Daut J. Inhibition of the sodium pump in guinea-pig ventricular muscle by dihydro-ouabain: effects of external potassium and sodium. J Physiol. 1983 Jun;339:643–662. doi: 10.1113/jphysiol.1983.sp014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. The role of intracellular sodium ions in the regulation of cardiac contractility. J Mol Cell Cardiol. 1982 Mar;14(3):189–192. doi: 10.1016/0022-2828(82)90119-5. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F., Reiter M. The alteration by propranolol of the inotropic and bathmotropic effects of dihydro-ouabain on guinea-pig papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jun;307(2):99–104. doi: 10.1007/BF00498450. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The relationship between sodium pump activity and twitch tension in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:475–494. doi: 10.1113/jphysiol.1980.sp013299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann E., Schoner W. Ouabain-receptor interactions in (Na+ + K+)-ATPase preparations. II. Effect of cations and nucleotides on rate constants and dissociation constants. Biochim Biophys Acta. 1973 Dec 22;330(3):302–315. doi: 10.1016/0005-2736(73)90235-6. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Bilezikian J. P., Loeb J. N. Kinetics and thermodynamics of ouabain binding by intact turkey erythrocytes: effects of external sodium ion, potassium ion, and temperature. J Gen Physiol. 1980 Oct;76(4):499–516. doi: 10.1085/jgp.76.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Grabowski W., Thielen J. Activation of the electrogenic sodium pump in guinea-pig atria by external potassium ions. J Physiol. 1978 Mar;276:515–524. doi: 10.1113/jphysiol.1978.sp012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H., Venetz K. Effects of Na and K ions on the active Na transport in guinea-pig auricles. Pflugers Arch. 1976 Sep 3;365(1):29–36. doi: 10.1007/BF00583625. [DOI] [PubMed] [Google Scholar]
- Halbach S., Schönsteiner G., Ebner F., Reiter M. The effects of p-chloromercuriphenylsulfonic acid (PCMBS) on force of contraction of mammalian myocardium and on ATP hydrolysis by sarcolemmal ATPase. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):121–129. doi: 10.1007/BF00508836. [DOI] [PubMed] [Google Scholar]
- Hobbs A. S., Dunham P. B. Interaction of external alkali metal ions with the Na-K pump of human erythrocytes: a comparison of their effects on activation of the pump and on the rate of ouabain binding. J Gen Physiol. 1978 Sep;72(3):381–402. doi: 10.1085/jgp.72.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki C., Lindenmayer G. E., Schwartz A. Effects of sodium and potassium on binding of ouabain to the transport adenosine triphosphatase. J Biol Chem. 1974 Aug 25;249(16):5135–5140. [PubMed] [Google Scholar]
- Kavaler F., Hyman P. M., Lefkowitz R. B. Positive and negative inotropic effects of elevated extracellular potassium level on mammalian ventricular muscle. J Gen Physiol. 1972 Sep;60(3):351–365. doi: 10.1085/jgp.60.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Lindenmayer G. E., Schwartz A. Nature of the transport adenosine triphosphatase digitalis complex. IV. Evidence that sodium-potassium competition modulates the rate of ouabain interaction iwth (Na + +K + ) adenosine triphosphatase during enzyme catalysis. J Biol Chem. 1973 Feb 25;248(4):1291–1300. [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Parker R. B., Waud D. R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J Pharmacol Exp Ther. 1971 Apr;177(1):1–12. [PubMed] [Google Scholar]
- Prindle K. H., Jr, Skelton C. L., Epstein S. E., Marcus F. I. Influence of extracellular potassium concentration on myocardial uptake and inotropic effect of tritiated digoxin. Circ Res. 1971 Mar;28(3):337–345. doi: 10.1161/01.res.28.3.337. [DOI] [PubMed] [Google Scholar]
- REITER M. DIE BEZIEHUNG VON CALCIUM UND NATRIUM ZUR INOTROPEN GLYKOSIDWIRKUNG. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1963 Sep 2;245:487–499. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Sodium-calcium exchange activity generates a current in cardiac membrane vesicles. Science. 1980 Jun 27;208(4451):1461–1464. doi: 10.1126/science.7384788. [DOI] [PubMed] [Google Scholar]
- Reiter M. Die Wertbestimmung inotrop wirkender Arzneimittel am isolierten Papillarmuskel. Arzneimittelforschung. 1967 Oct;17(10):1249–1253. [PubMed] [Google Scholar]
- Reiter M., Seibel K., Stickel F. J. Sodium dependence of the inotropic effect of a reduction in extracellular potassium concentration. Naunyn Schmiedebergs Arch Pharmakol. 1971;268(4):361–378. doi: 10.1007/BF00997062. [DOI] [PubMed] [Google Scholar]
- Reiter M., Stickel F. J., Weber S. The influence of the extracellular potassium concentration on the glycoside effects upon contractile force and action potential duration of the guinea- pig papillary muscle. Experientia. 1966 Oct 15;22(10):665–666. doi: 10.1007/BF01902431. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling W., Ebner F., Reiter M. The opposite effects of magnesium and calcium on the contraction of the guinea-pig ventricular myocardium in dependence on the sodium concentration. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jun;303(2):111–119. doi: 10.1007/BF00508056. [DOI] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Relation between intracellular sodium and twitch tension in sheep cardiac Purkinje strands exposed to cardiac glycosides. Circ Res. 1983 Jun;52(6):697–705. doi: 10.1161/01.res.52.6.697. [DOI] [PubMed] [Google Scholar]


