Abstract
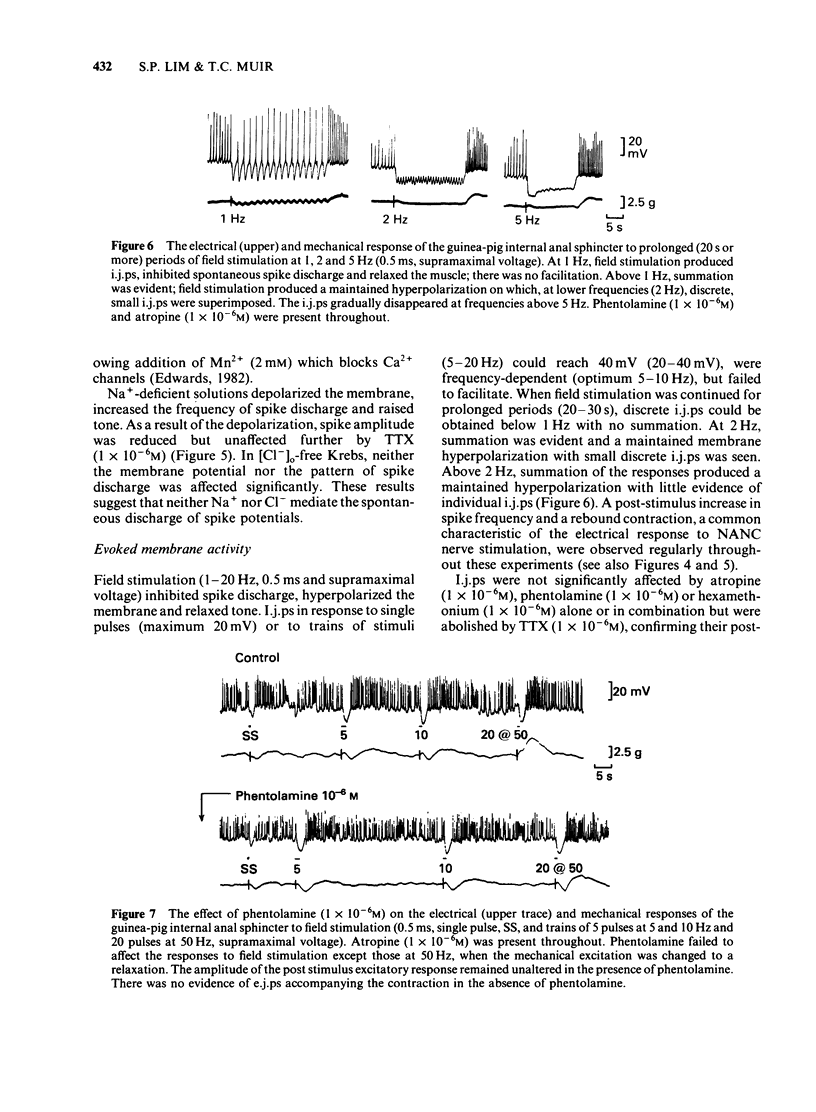
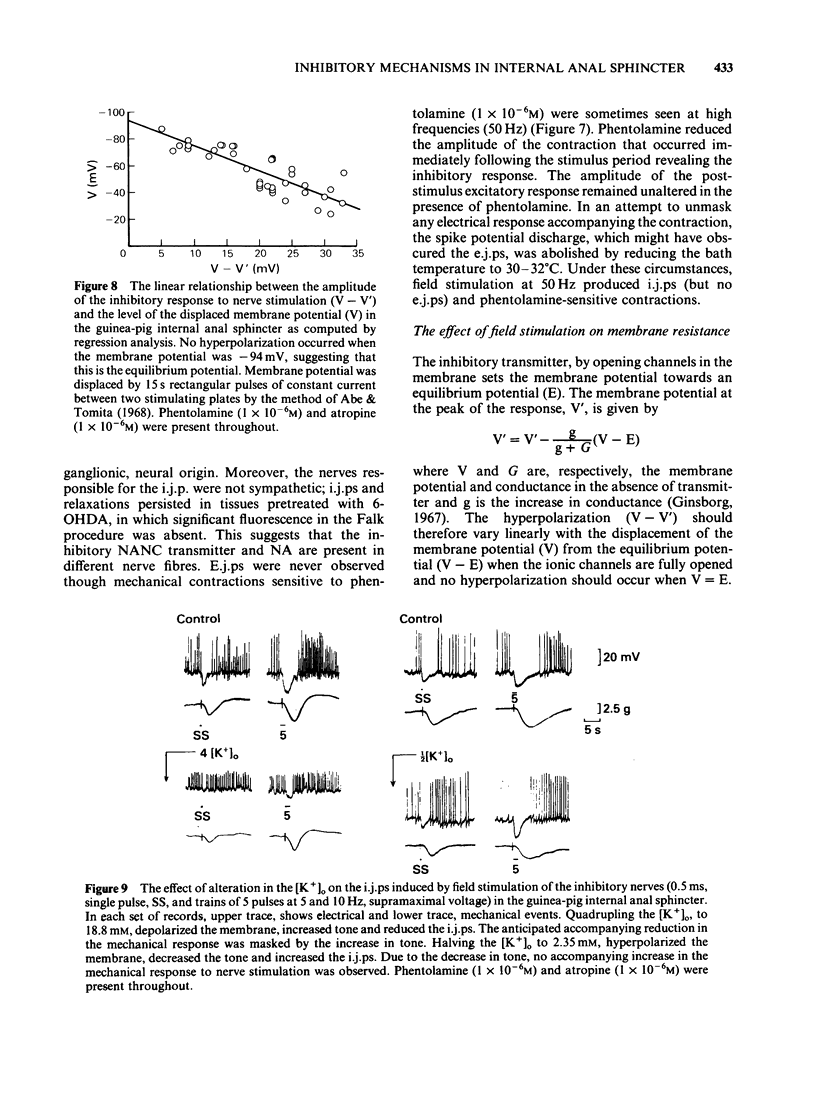
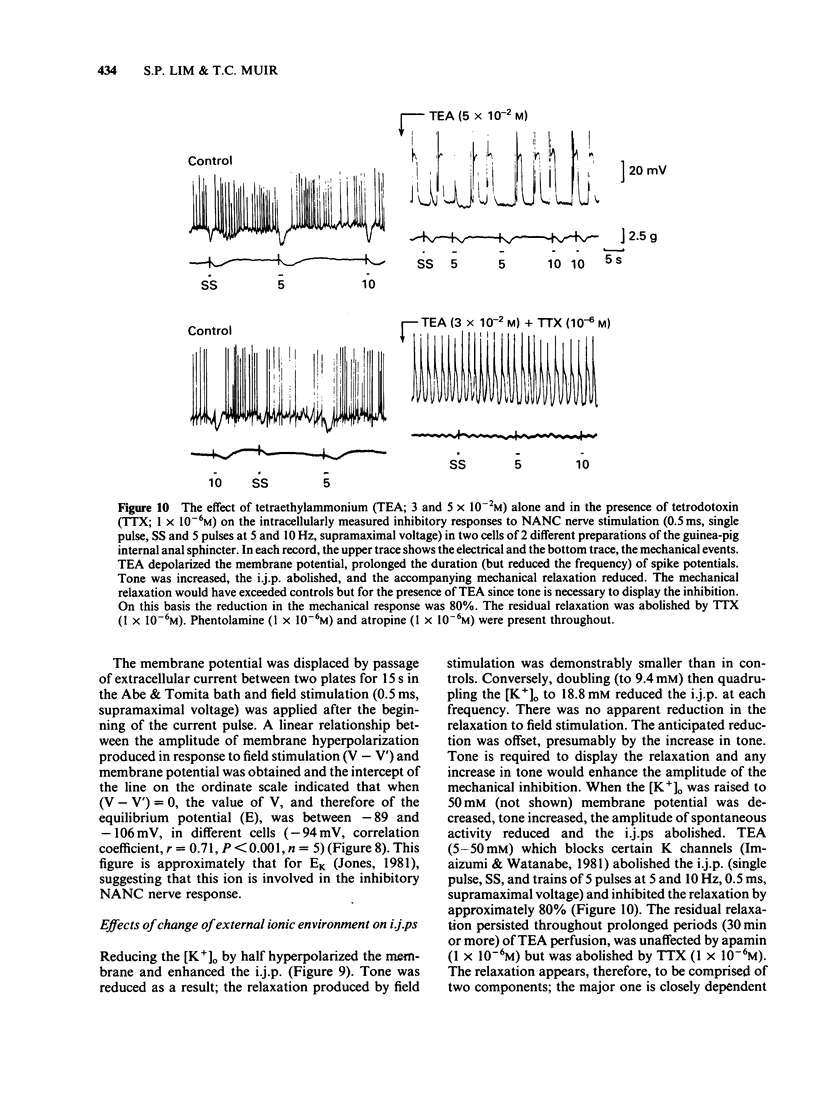
The electrical membrane characteristics and the response of the circular muscle of the guinea-pig internal anal sphincter (i.a.s.) to field stimulation were studied in vitro using intracellular microelectrodes and conventional mechanical recording techniques. The i.a.s. developed its own tone (3-4 g), following initial stretch (1 g) and spontaneous spike potentials were evident. In the absence of spike potentials, tone declined and disappeared. Tone was not significantly reduced by phentolamine (1 X 10(-6)M). The resting membrane potential, measured between spontaneous spike potentials, was -45 +/- 3.0 mV (n = 224); the space constant (lambda) was 1.13 +/- 0.1 mm (n = 13). Spikes usually overshot by approximately 15 mV. The frequency of spike potential discharge (1-3 Hz) varied with the degree of membrane depolarization, being increased in K+-rich and decreased in K+-deficient solutions or by the presence of Mn2+. It was not significantly affected by C1-withdrawal but was increased in Na+-deficient solutions with or without tetrodotoxin (TTX; 1 X 10(-6)M). Field stimulation (1-20 Hz, 0.5 ms, supramaximal voltage) produced inhibitory junction potentials (i.j.ps) and relaxed tone; at high frequencies (50 Hz or greater), contractions were observed but excitatory junction potentials (e.j.ps) were not. I.j.ps and relaxations were inhibited by apamin (1 X 10(-6)M), TTX (1 X 10(-6)M) but not by atropine (1 X 10(-6)M), phentolamine (1 X 10(-6)M) or hexamethonium (1 X 10(-6)M). I.j.ps were reduced by hyperpolarization and enhanced by depolarization of the membrane by current pulses (15s). The mean equilibrium potential for the i.j.p. was -94 mV (correlation coefficient, gamma = 0.71, n = 5, p less than 0.001). I.j.ps were enhanced in K+-deficient solutions and reduced in K+-rich solutions. Together these results suggest that the i.j.p. is mediated by an increased GK. The absence of [Ca2+]o or the presence of Mn2+ (2 mM) abolished the i.j.p.; in contrast Na+-deficient or C1-free solutions were ineffective in this respect. Tetraethylammonium (5-50mM) abolished the i.j.p.; the accompanying relaxation was reduced by about 80%. The major aspect of the relaxation to nerve stimulation is mediated by membrane hyperpolarization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Muir T. C. Electrical and mechanical responses of the bovine retractor penis to nerve stimulation and to drugs. J Auton Pharmacol. 1984 Dec;4(4):261–271. doi: 10.1111/j.1474-8673.1984.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Muir T. C. Mechanisms underlying electrical and mechanical responses of the bovine retractor penis to inhibitory nerve stimulation and to an inhibitory extract. Br J Pharmacol. 1985 May;85(1):149–161. doi: 10.1111/j.1476-5381.1985.tb08842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W., Daniel E. E. Comparative study of the smooth muscle layers of the rabbit duodenum. J Physiol. 1980 Dec;309:13–27. doi: 10.1113/jphysiol.1980.sp013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. The selectivity of ion channels in nerve and muscle. Neuroscience. 1982 Jun;7(6):1335–1366. doi: 10.1016/0306-4522(82)90249-4. [DOI] [PubMed] [Google Scholar]
- Furness J. B. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969 Dec;205(3):549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The ramifications of adrenergic nerve terminals in the rectum, anal sphincter and anal accessory muscles of the guinea-pig. Z Anat Entwicklungsgesch. 1973 May 30;140(1):109–128. doi: 10.1007/BF00520721. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Muir T. C. Species and tissue variation in extraneuronal and neuronal accumulation of noradrenaline. J Physiol. 1970 Mar;206(3):591–604. doi: 10.1113/jphysiol.1970.sp009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol. 1981 Jul;316:33–46. doi: 10.1113/jphysiol.1981.sp013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. Membrane properties and inhibitory innervation of the circular muscle cells of guinea-pig caecum. J Physiol. 1973 Jun;231(3):455–470. doi: 10.1113/jphysiol.1973.sp010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A., Jager L. P. Ion fluxes during the inhibitory junction potential in the guinea-pig taenia coli. J Physiol. 1975 Sep;250(3):681–691. doi: 10.1113/jphysiol.1975.sp011077. [DOI] [PMC free article] [PubMed] [Google Scholar]


