Abstract
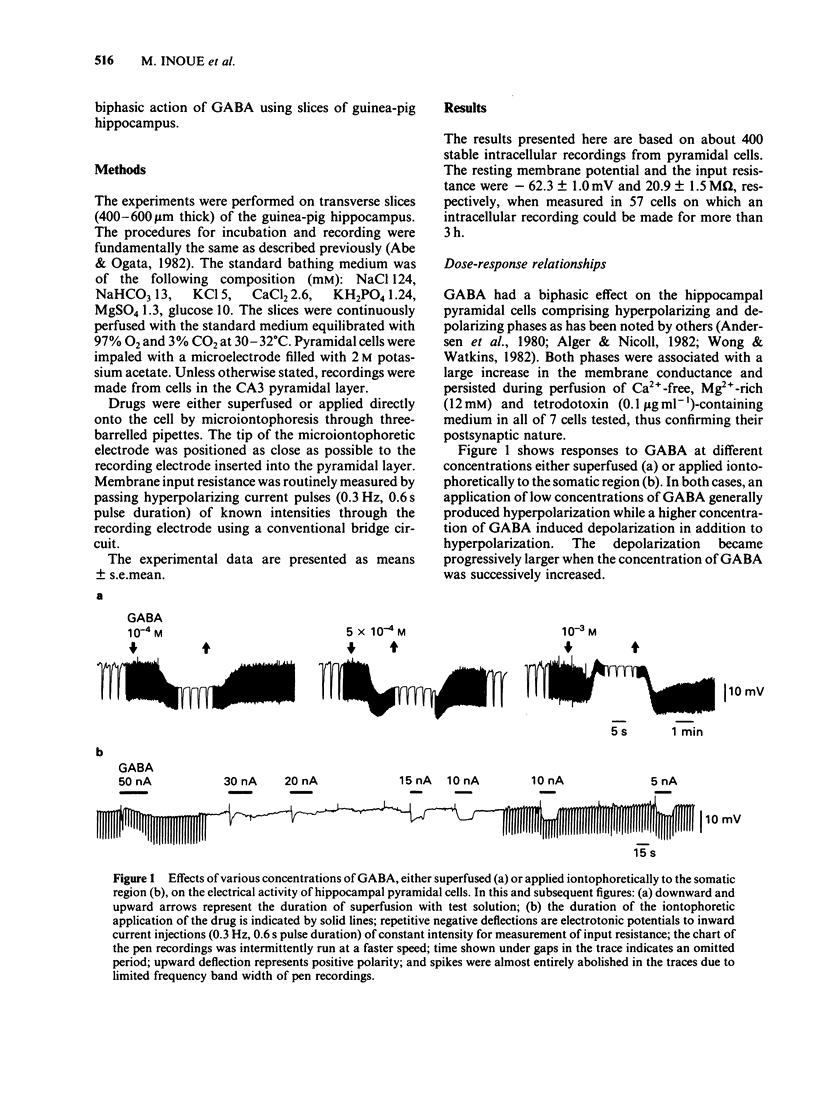
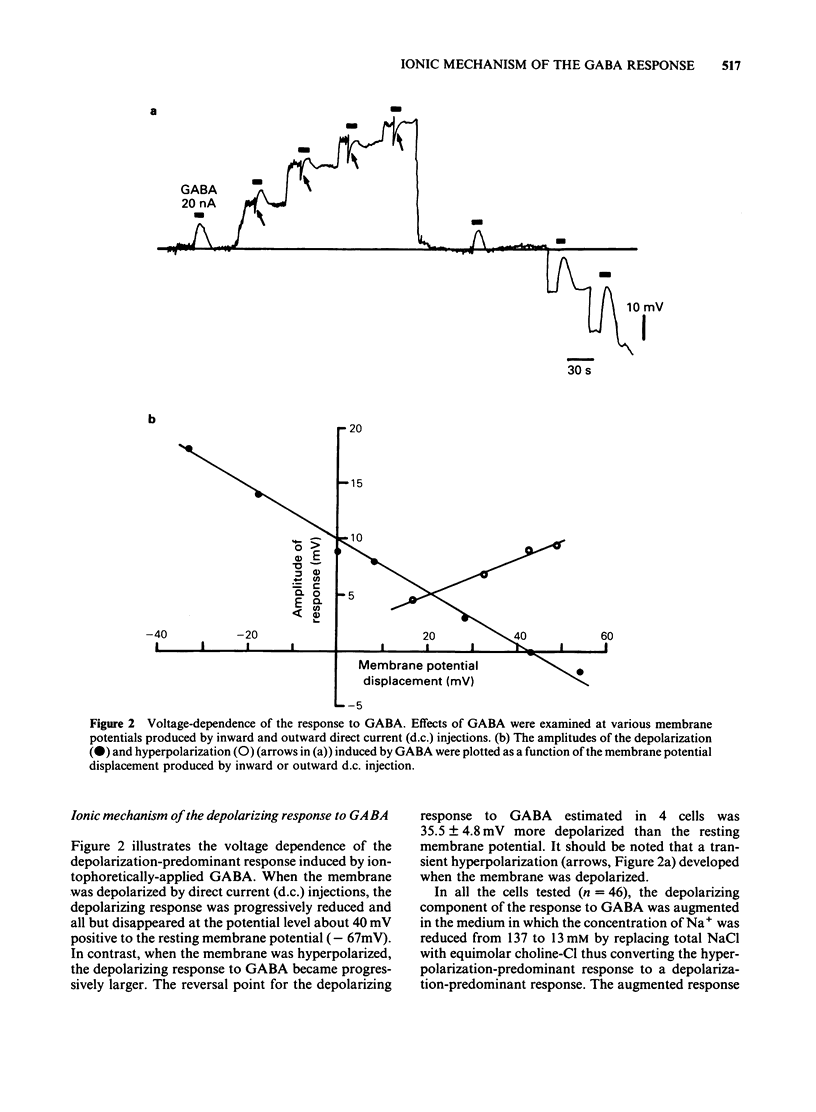
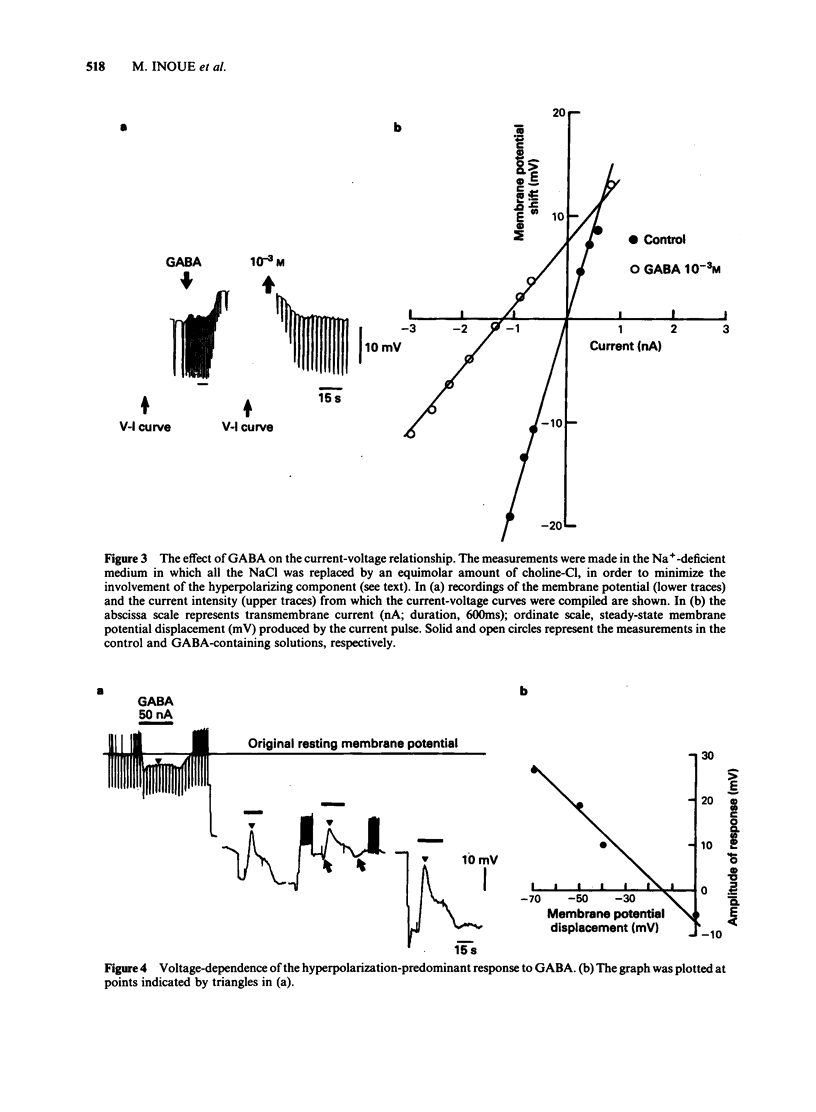
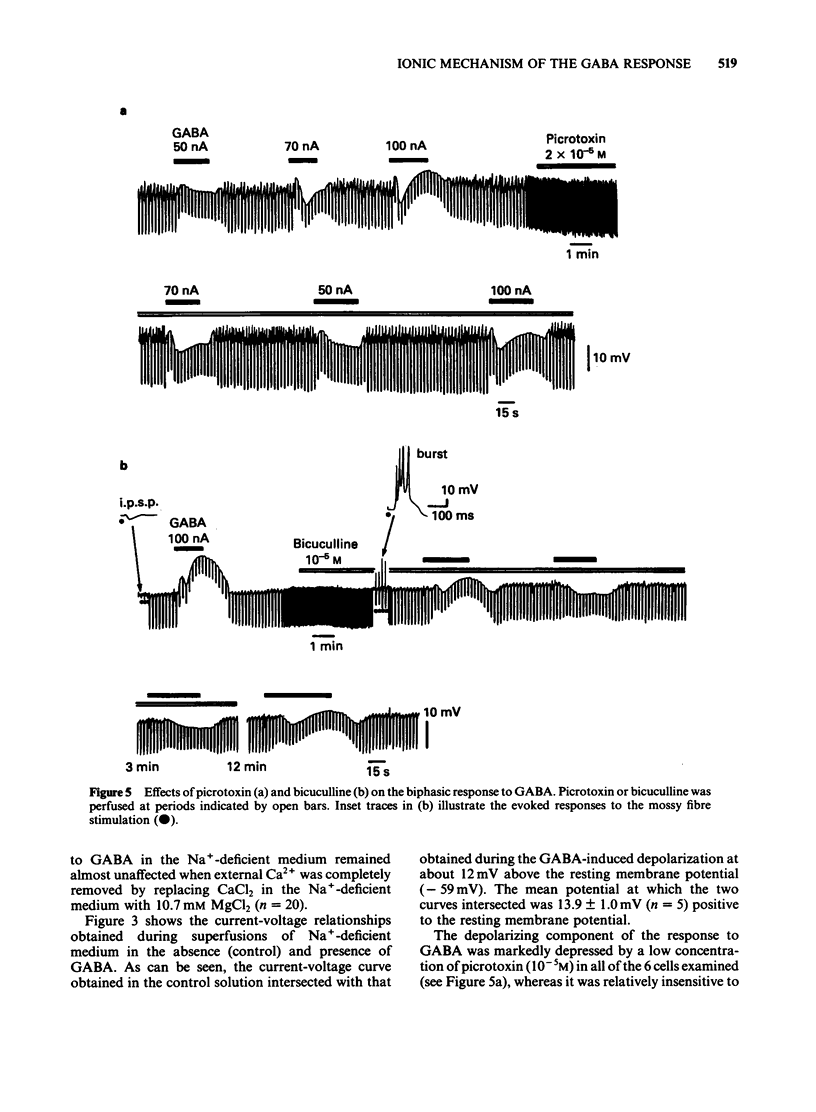
The mechanism underlying the action of gamma-aminobutyric acid (GABA) in the hippocampus was investigated using guinea-pig brain slices. GABA either superfused or applied directly by microiontophoresis produced a biphasic response in pyramidal cells, comprising hyperpolarizing and depolarizing components. When different concentrations of GABA were applied to the same neurone, the lower concentrations generally produced a hyperpolarization-predominant response, while higher concentrations resulted in a depolarization-predominant response. The depolarizing component of the response to GABA was augmented in a medium containing a low concentration of Cl-, relatively unaffected by a change in external K+ concentration, and blocked by picrotoxin (2 X 10(-5) M). The depolarizing response to GABA persisted in a Ca2+-free medium in which the concentration of Na+ was reduced to 13 mM. Combined application of low doses of picrotoxin and bicuculline eliminated the major part of the depolarizing component of the biphasic response to GABA and produced a relatively pure hyperpolarizing response. The reversal potential of this pharmacologically 'isolated' hyperpolarizing response to GABA was estimated, from the current-voltage relationships, to be about -90 mV and was the same as that of the hyperpolarization induced by baclofen. When the membrane was successively hyperpolarized by inward direct current (d.c.) injections, the reversal point of the 'pharmacologically isolated' hyperpolarizing response to GABA coincided with that of the post-burst hyperpolarization. Low concentrations of Cl- in the bathing medium had no noticeable effect on the hyperpolarizing component of the response to GABA, whereas it markedly increased the amplitude of the depolarizing component. These results suggest that the action of GABA in the hippocampus may involve an activation of K+ conductance.
Full text
PDF
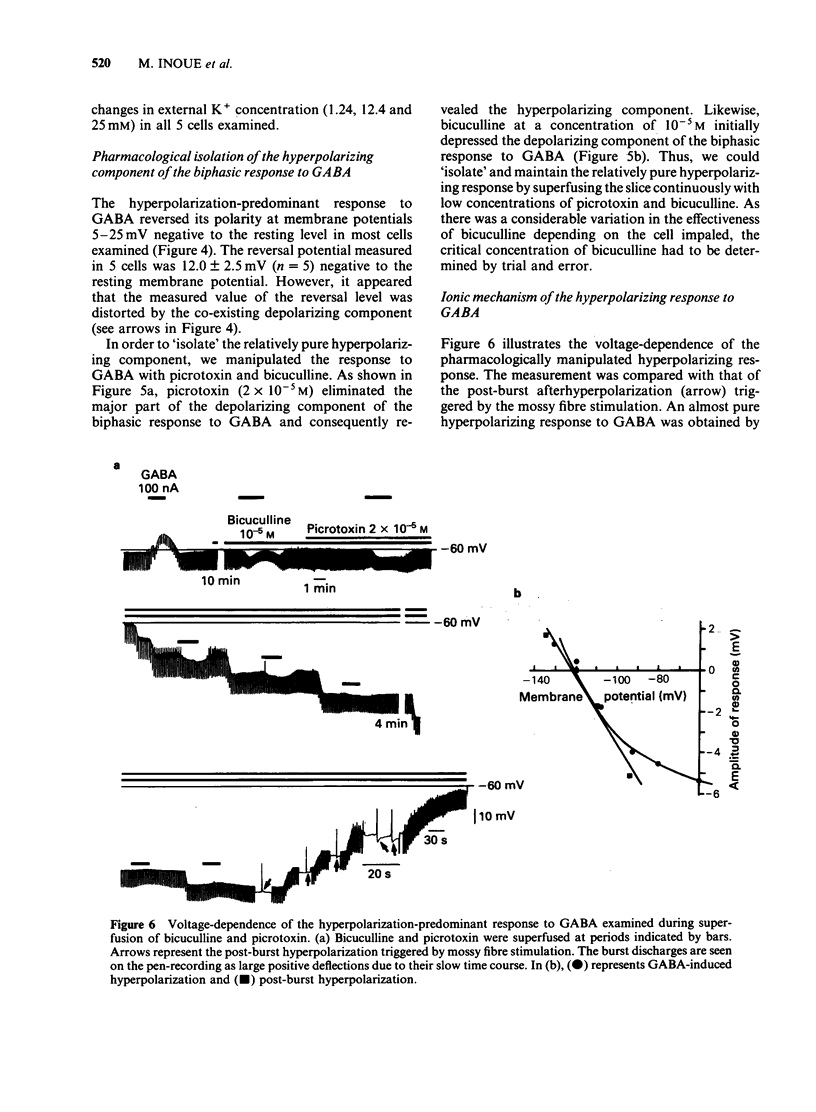
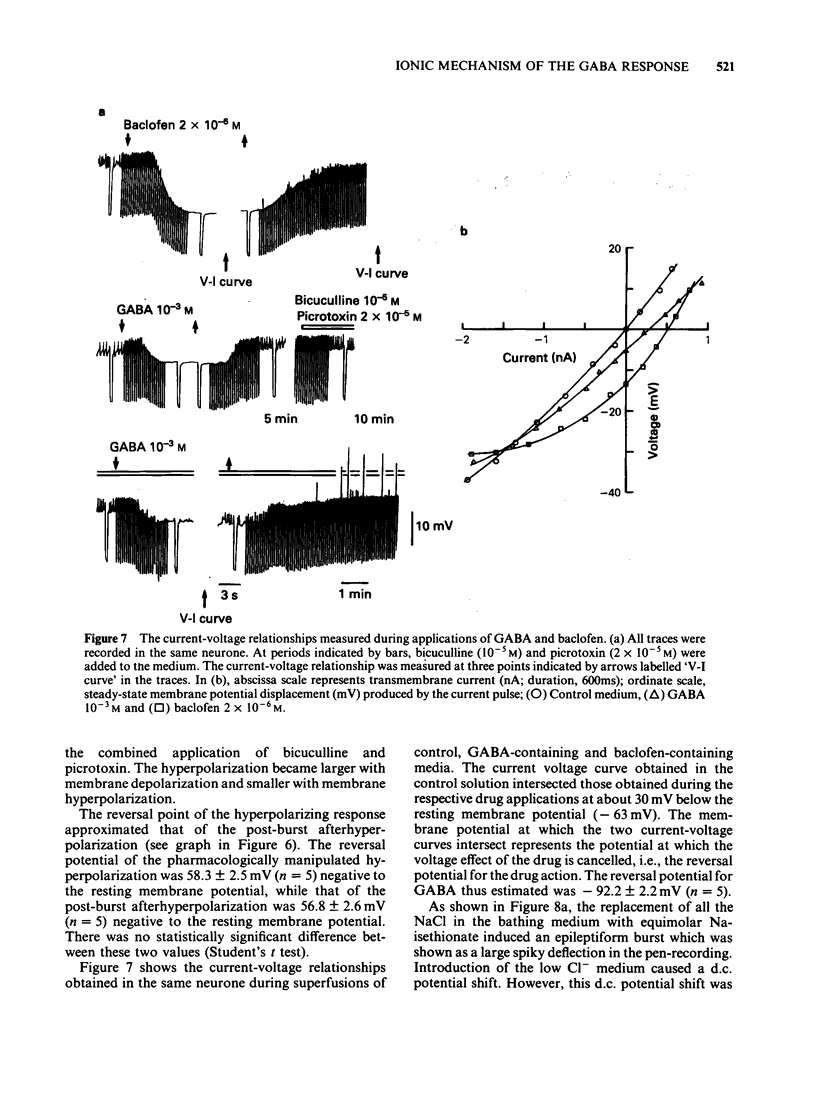
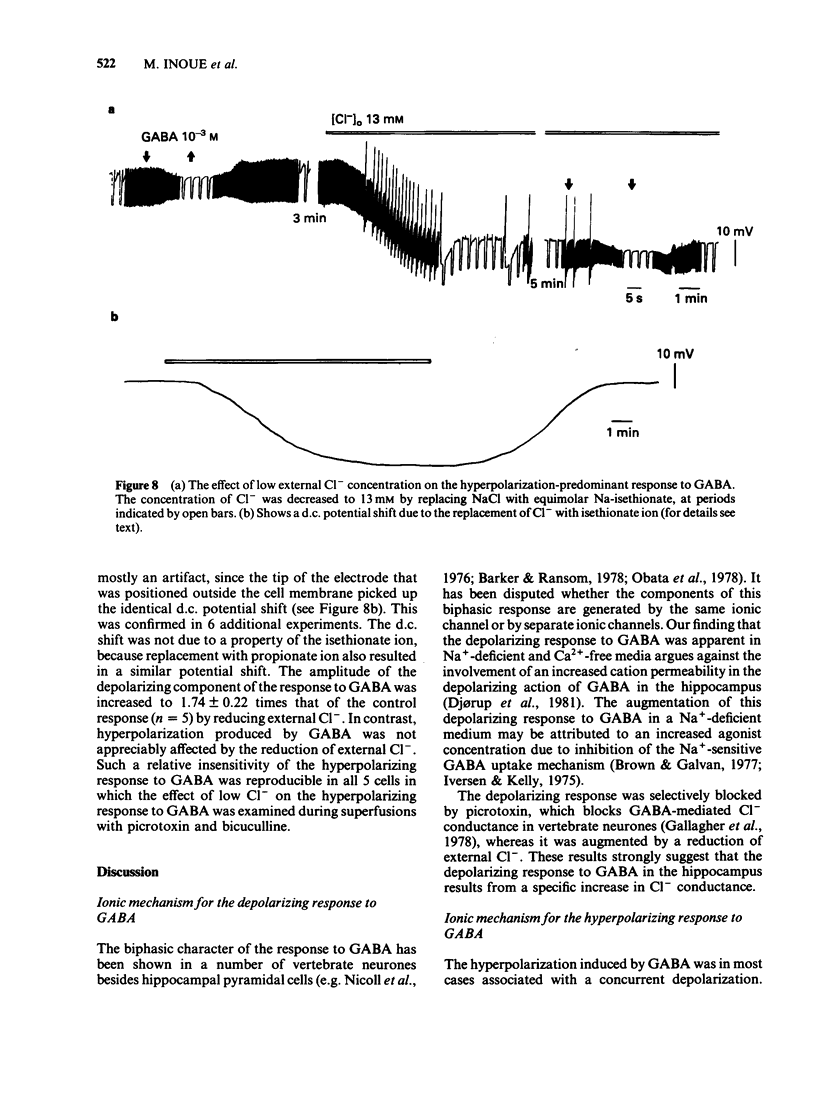


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982 Jun;327:157–171. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bührle C. P., Sonnhof U. Intracellular ion activities and equilibrium potentials in motoneurones and glia cells of the frog spinal cord. Pflugers Arch. 1983 Feb;396(2):144–153. doi: 10.1007/BF00615519. [DOI] [PubMed] [Google Scholar]
- Djørup A., Jahnsen H., Laursen A. M. The dendritic response to GABA in CA1 of the hippocampal slice. Brain Res. 1981 Aug 24;219(1):196–201. doi: 10.1016/0006-8993(81)90282-1. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Baclofen activates voltage-dependent and 4-aminopyridine sensitive K+ conductance in guinea-pig hippocampal pyramidal cells maintained in vitro. Br J Pharmacol. 1985 Apr;84(4):833–841. doi: 10.1111/j.1476-5381.1985.tb17377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A., Barker J. L. Analysis of amino acid responses on frog motoneurones. Neuropharmacology. 1976 Jan;15(1):45–53. doi: 10.1016/0028-3908(76)90096-4. [DOI] [PubMed] [Google Scholar]
- Obata K., Oide M., Tanaka H. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Res. 1978 Apr 7;144(1):179–184. doi: 10.1016/0006-8993(78)90447-x. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Localization of transmitter candidates in the brain: the hippocampal formation as a model. Prog Neurobiol. 1977;8(2):119–181. doi: 10.1016/0301-0082(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Watkins D. J. Cellular factors influencing GABA response in hippocampal pyramidal cells. J Neurophysiol. 1982 Oct;48(4):938–951. doi: 10.1152/jn.1982.48.4.938. [DOI] [PubMed] [Google Scholar]


