Abstract
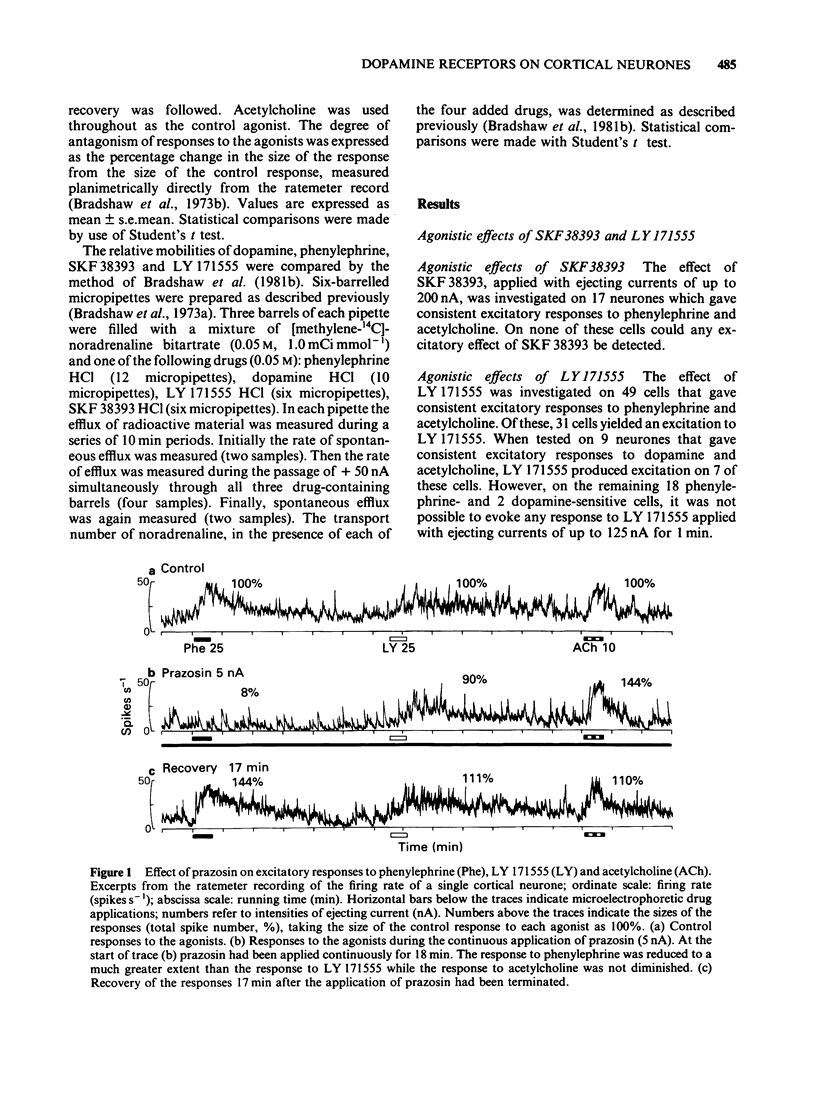
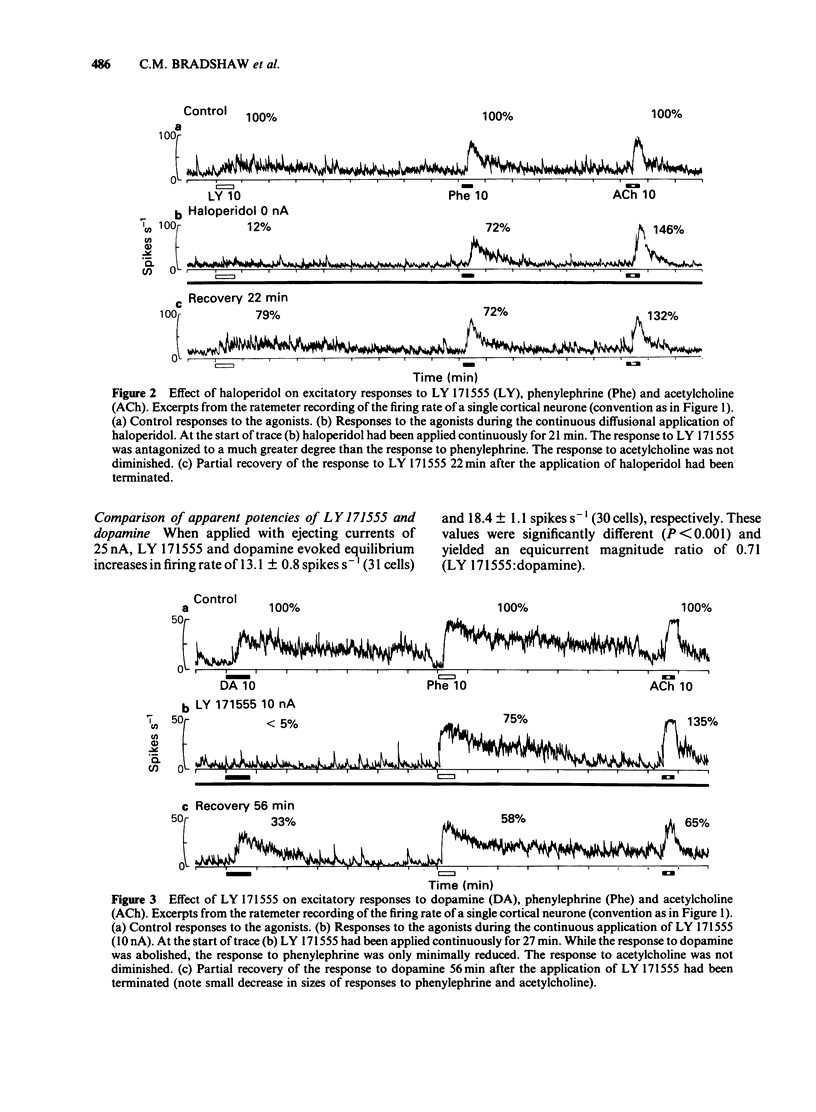
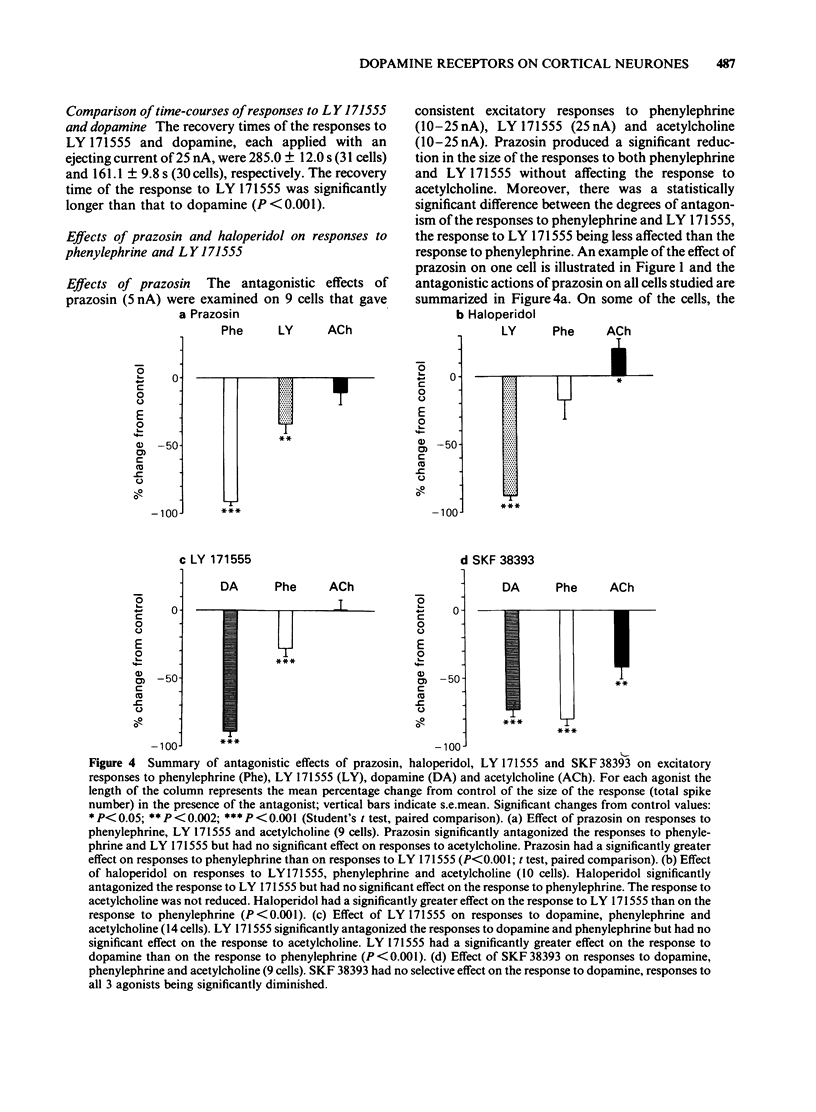
The technique of microelectrophoresis was used to evaluate the relative contribution of D1 and D2 dopamine receptors towards the mediation of the excitatory response of single neurones to dopamine in the somatosensory cortex of the rat. The selective D1 dopamine receptor agonist, SKF 38393, failed to excite any of the cells to which it was applied. In contrast, the selective D2 dopamine receptor agonist, LY 171555, excited the majority of cells tested. The apparent potency of LY 171555 was significantly lower than that of dopamine. When the mobilities of SKF 38393 and LY 171555 were assessed by an in vitro method, they were found to be at least as great as those of dopamine and phenylephrine, suggesting that the lack of effect of SKF 38393 and the lower apparent potency of LY 171555 compared to dopamine reflect genuine biological phenomena. The alpha 1-adrenoceptor antagonist, prazosin, discriminated between excitatory responses to the alpha 1-adrenoceptor agonist, phenylephrine, and LY 171555: responses to phenylephrine were more susceptible to antagonism than were those to LY 171555. The dopamine receptor antagonist, haloperidol, produced the reverse discrimination: responses to LY 171555 were more affected than were those to phenylephrine. Neither antagonist reduced the response to the control agonist, acetylcholine. When applied continuously with low ejecting currents, LY 171555 antagonized the excitatory response to dopamine while the response to phenylephrine was relatively preserved. The response to acetylcholine was unaffected. When similarly applied, SKF 38393 had no selective action on the response to dopamine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan P., Bradshaw C. M., Pun R. Y., Slater N. T., Szabadi E. Responses of single cortical neurones to noradrenaline and dopamine. Neuropharmacology. 1978 Aug;17(8):611–617. doi: 10.1016/0028-3908(78)90156-9. [DOI] [PubMed] [Google Scholar]
- Bevan P., Bradshaw C. M., Szabadi E. The pharmacology of adrenergic neuronal responses in the cerebral cortex: evidence for excitatory alpha- and inhibitory beta-receptors. Br J Pharmacol. 1977 Apr;59(4):635–641. doi: 10.1111/j.1476-5381.1977.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. M., Pun R. Y., Slater N. T., Stoker M. J., Szabadi E. Differential antagonistic effects of haloperidol on excitatory responses of cortical neurones to phenylephrine, noradrenaline and dopamine. Neuropharmacology. 1983 Aug;22(8):945–952. doi: 10.1016/0028-3908(83)90210-1. [DOI] [PubMed] [Google Scholar]
- Bradshaw C. M., Pun R. Y., Slater N. T., Szabadi E. A procedure for comparing the mobilities of unlabeled drugs used in microelectrophoresis experiments. J Pharmacol Methods. 1981 Jan;5(1):67–73. doi: 10.1016/0160-5402(81)90104-2. [DOI] [PubMed] [Google Scholar]
- Bradshaw C. M., Pun R. Y., Slater N. T., Szabadi E. Comparison of the effects of methoxamine with those of noradrenaline and phenylephrine on single cerebral cortical neurones. Br J Pharmacol. 1981 May;73(1):47–54. doi: 10.1111/j.1476-5381.1981.tb16770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. M., Roberts M. H., Szabadi E. Kinetics of the release of noradrenaline from micropipettes: interaction between ejecting and retaining currents. Br J Pharmacol. 1973 Dec;49(4):667–677. doi: 10.1111/j.1476-5381.1973.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. M., Szabadi E. A technique for achieving greater stability of the brain for microiontophoretic studies of single cortical neurones. Br J Pharmacol. 1972 May;45(1):185P–186P. [PMC free article] [PubMed] [Google Scholar]
- Christensen A. V., Arnt J., Hyttel J., Larsen J. J., Svendsen O. Pharmacological effects of a specific dopamine D-1 antagonist SCH 23390 in comparison with neuroleptics. Life Sci. 1984 Apr 16;34(16):1529–1540. doi: 10.1016/0024-3205(84)90607-6. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Beaulieu M., Itoh Y. Pharmacological and biochemical evidence for the existence of two categories of dopamine receptor. Can J Neurol Sci. 1984 Feb;11(1 Suppl):114–117. doi: 10.1017/s0317167100046254. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Plantjé J. F., Hansen H. A., Daus F. J., Stoof J. C. The effects of SCH 23390, YM 09151-2, (+)- and (-)-3-PPP and some classical neuroleptics on D-1 and D-2 receptors in rat neostriatum in vitro. Eur J Pharmacol. 1984 Oct 1;105(1-2):73–83. doi: 10.1016/0014-2999(84)90650-2. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Setler P. E., Sarau H. M., Zirkle C. L., Saunders H. L. The central effects of a novel dopamine agonist. Eur J Pharmacol. 1978 Aug 15;50(4):419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Szabadi E., Bradshaw C. M. The role of physical and biological factors in determining the time course of neuronal responses. Neuropharmacology. 1974 Jun;13(6):537–545. doi: 10.1016/0028-3908(74)90143-9. [DOI] [PubMed] [Google Scholar]
- Tsuruta K., Frey E. A., Grewe C. W., Cote T. E., Eskay R. L., Kebabian J. W. Evidence that LY-141865 specifically stimulates the D-2 dopamine receptor. Nature. 1981 Jul 30;292(5822):463–465. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]


