Abstract
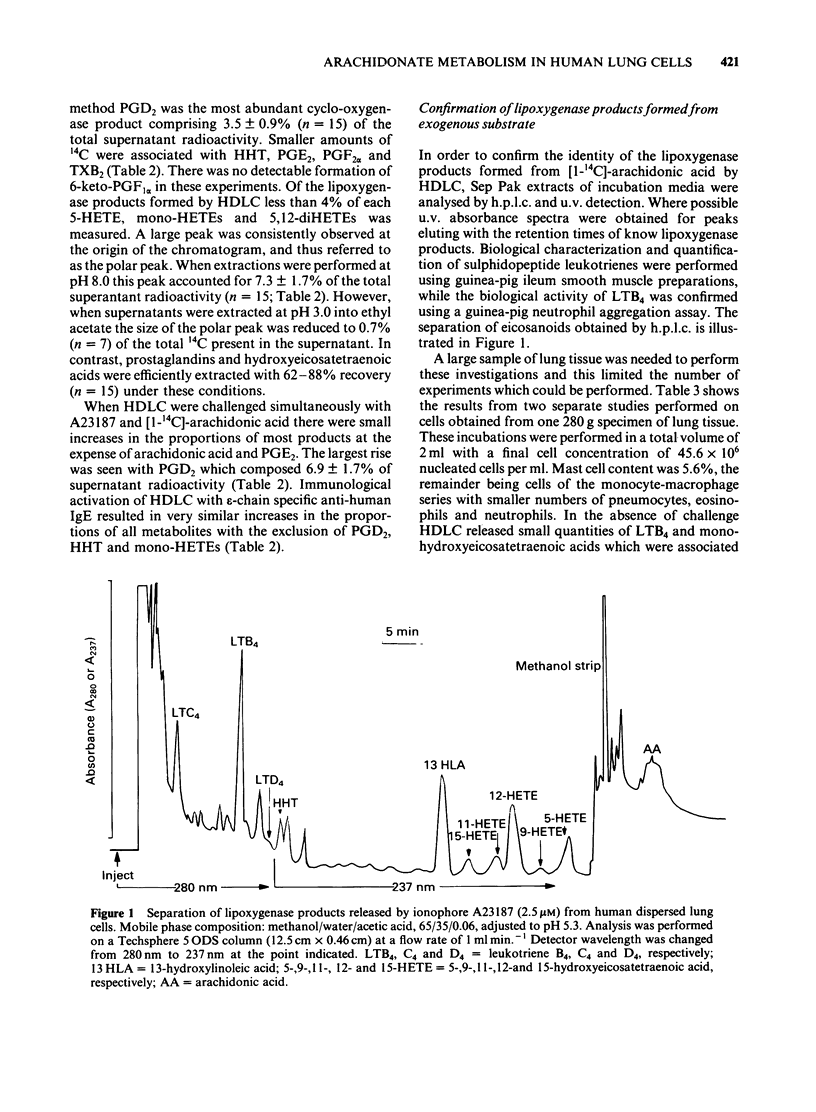
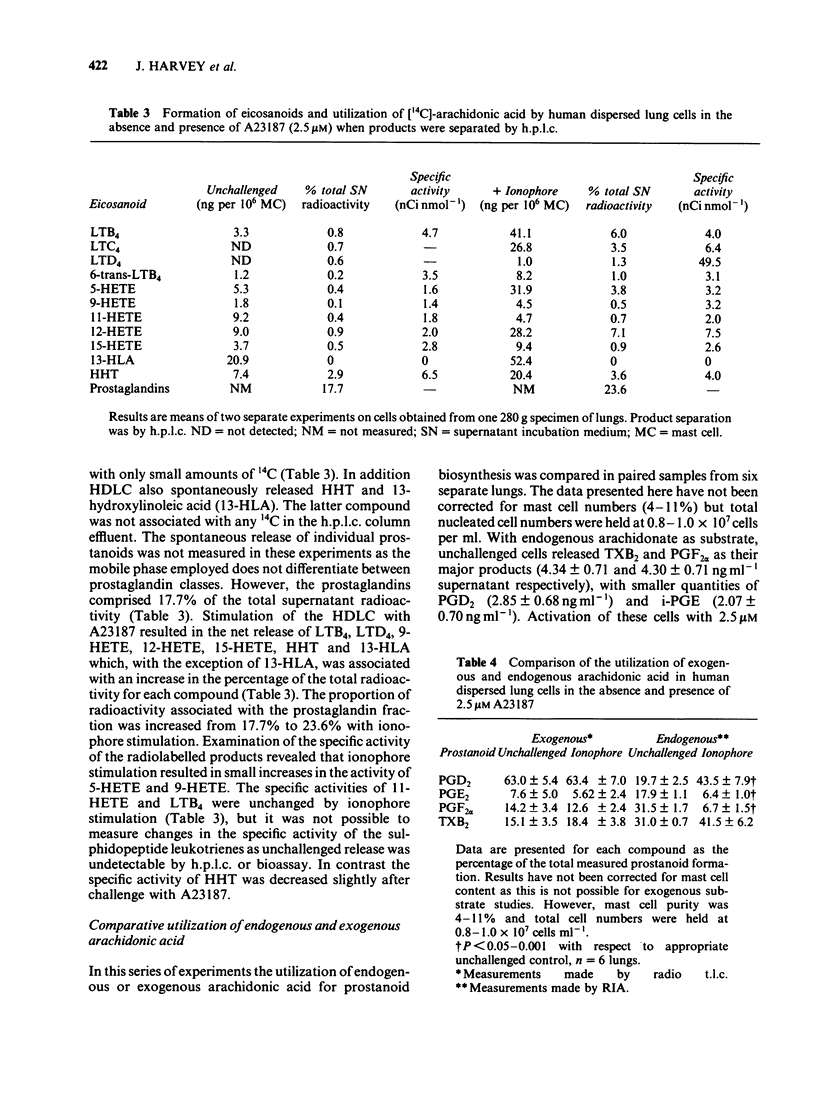
Eicosanoid release from human dispersed lung cells (HDLC) containing ca 5% mast cells was studied before and after cell activation with ionophore A23187 or anti-IgE. Basal release of eicosanoids synthesized from endogenous arachidonate was measured by radioimmunoassay. In descending order of abundance the products were: 5-hydroxyeicosatetraenoic acid (5-HETE) greater than thromboxane B2 (TXB2) greater than prostaglandin F2 alpha (PGF2 alpha) approximately immunoreactive (i)-PGE2 greater than PGD2 greater than 6-keto-PGF1 alpha approximately i-LTC4. Stimulation of HDLC with ionophore A23187 or, after passive sensitization, with anti-IgE resulted in 2-10 fold increases in the generation of individual eicosanoids. In terms of net generation the most abundant products were PGD2 and TXB2 with either stimulus. Activation with A23187 caused net release of i-LTC4 and 5-HETE, but these products were not measured after immunological activation. A more complete profile of lipoxygenase products released from HDLC dispersed from one lung was obtained after separation by high performance liquid chromatography combined with ultra violet spectroscopy and bioassay. The major products released from the cells from this lung with ionophore stimulation were 13-hydroxylinoleic acid greater than LTB4 greater than 5-HETE greater than 12-HETE greater than LTC4 greater than 15-HETE greater than 11-HETE approximately 9-HETE. When the utilization of exogenous [14C]-arachidonic acid for prostanoid biosynthesis was compared to that of endogenous unlabelled arachidonate the formation of TXB2 was consistently underestimated. These results imply compartmentalization of arachidonic acid utilization in Ca2+-activated HDLC. In unstimulated cells the proportional formation of PGD2 was overestimated when exogenous arachidonic acid was substrate. After activation with A23187 the proportions of PGD2 were similar with both substrate sources. The large proportions of PGD2 and TXB2 generated by HDLC further supports the view that these eicosanoids may be important inflammatory mediators in lung tissue.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ubaidi F., Bakhle Y. S. Differences in biological activation of arachidonic acid in perfused lungs from guinea pig, rat and man. Eur J Pharmacol. 1980 Mar 7;62(1):89–96. doi: 10.1016/0014-2999(80)90484-7. [DOI] [PubMed] [Google Scholar]
- Ally A. I., Boucher R., Knowles M. R., Eling T. E. Metabolism of prostaglandin endoperoxide by microsomes from human lung parenchyma and comparison with metabolites produced by pig, bovine, rat, mouse and guinea-pig. Prostaglandins. 1982 Oct;24(4):575–584. doi: 10.1016/0090-6980(82)90015-6. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Smith H. W., Fitzpatrick F. A., Sun F. F., McGuire J. C. 6,9-deepoxy-6,9,-(phenylimino)-delta 6,8-prostaglandin I1, (U-60,257), a new inhibitor of leukotriene C and D synthesis: in vitro studies. Prostaglandins. 1982 May;23(5):759–771. [PubMed] [Google Scholar]
- Baker S. R., Boot J. R., Jamieson W. B., Osborne D. J., Sweatman W. J. The comparative in vitro pharmacology of leukotriene D4 and its isomers. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1258–1264. doi: 10.1016/0006-291x(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Hansson G., Hedqvist P., Björck T., Granström E., Dahlén B. Allergen challenge of lung tissue from asthmatics elicits bronchial contraction that correlates with the release of leukotrienes C4, D4, and E4. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1712–1716. doi: 10.1073/pnas.80.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr, Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J Immunol. 1983 Dec;131(6):2965–2976. [PubMed] [Google Scholar]
- Hardy C. C., Robinson C., Tattersfield A. E., Holgate S. T. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984 Jul 26;311(4):209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- Harvey J., Osborne D. J. A rapid method for detecting inhibitors of both cyclo-oxygenase lipoxygenase metabolites of arachidonic acid. J Pharmacol Methods. 1983 Apr;9(2):147–155. doi: 10.1016/0160-5402(83)90006-2. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Burns G. B., Robinson C., Church M. K. Anaphylactic- and calcium-dependent generation of prostaglandin D2 (PGD2), thromboxane B2, and other cyclooxygenase products of arachidonic acid by dispersed human lung cells and relationship to histamine release. J Immunol. 1984 Oct;133(4):2138–2144. [PubMed] [Google Scholar]
- Hopkins N. K., Sun F. F., Gorman R. R. Thromboxane A2 biosynthesis in human lung fibroblasts WI-38. Biochem Biophys Res Commun. 1978 Nov 29;85(2):827–836. doi: 10.1016/0006-291x(78)91237-8. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Desai U., Gonzalez-Crussi F., Lamb R., Chu A. Two phospholipase pools for prostaglandin synthesis in macrophages. Nature. 1981 Apr 23;290(5808):710–713. doi: 10.1038/290710a0. [DOI] [PubMed] [Google Scholar]
- Jose P. J., Seale J. P. Incorporation and metabolism of [14C]-arachidonic acid in guinea-pig lungs. Br J Pharmacol. 1979 Dec;67(4):519–526. doi: 10.1111/j.1476-5381.1979.tb08697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Drazen J. M., Clark D. A., Marfat A., Corey E. J. Slow reacting substances of anaphylaxis: identification of leukotrienes C-1 and D from human and rat sources. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3710–3714. doi: 10.1073/pnas.77.6.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Soter N. A., Diamond P. T., Austen K. F., Oates J. A., Roberts L. J., 2nd Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982 Oct;129(4):1627–1631. [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Schleimer R. P., Peters S. P., Schulman E. S., Adams G. K., 3rd, Newball H. H., Lichtenstein L. M. Generation of leukotrienes by purified human lung mast cells. J Clin Invest. 1982 Oct;70(4):747–751. doi: 10.1172/JCI110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Piper P. J., Taylor G. W., Tippins J. R. Comparative studies on immunologically and non-immunologically produced slow-reacting substances from man, guinea-pig and rat. Br J Pharmacol. 1979 Oct;67(2):179–184. doi: 10.1111/j.1476-5381.1979.tb08664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Jones C. M., Piper P. J., Samhoun M. N., Tippins J. R. Slow reacting substances (leukotrienes): enzymes involved in their biosynthesis. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4838–4842. doi: 10.1073/pnas.79.16.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne D. J., Peters B. J., Meade C. J. The separation of leukotrienes and hydroxyeicosatetraenoic acid metabolites of arachidonic acid by high performance liquid chromatography (HPLC). Prostaglandins. 1983 Nov;26(5):817–832. doi: 10.1016/0090-6980(83)90065-5. [DOI] [PubMed] [Google Scholar]
- Paterson N. A., Wasserman S. I., Said J. W., Austen K. F. Release of chemical mediators from partially purified human lung mast cells. J Immunol. 1976 Oct;117(4):1356–1362. [PubMed] [Google Scholar]
- Peters S. P., MacGlashan D. W., Jr, Schulman E. S., Schleimer R. P., Hayes E. C., Rokach J., Adkinson N. F., Jr, Lichtenstein L. M. Arachidonic acid metabolism in purified human lung mast cells. J Immunol. 1984 Apr;132(4):1972–1979. [PubMed] [Google Scholar]
- Piper P. J., Walker J. L. The release of spasmogenic substances from human chopped lung tissue and its inhibition. Br J Pharmacol. 1973 Feb;47(2):291–304. doi: 10.1111/j.1476-5381.1973.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Holgate S. T. Mast cell-dependent inflammatory mediators and their putative role in bronchial asthma. Clin Sci (Lond) 1985 Feb;68(2):103–112. doi: 10.1042/cs0680103. [DOI] [PubMed] [Google Scholar]
- Robinson C., Hoult J. R. Evidence for functionally distinct pools of phospholipase responsible for prostaglandin release from the perfused guinea-pig lung. Eur J Pharmacol. 1980 Jun 27;64(4):333–339. doi: 10.1016/0014-2999(80)90241-1. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Schulman E. S., MacGlashan D. W., Jr, Peters S. P., Hayes E. C., Adams G. K., 3rd, Lichtenstein L. M., Adkinson N. F., Jr Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest. 1983 Jun;71(6):1830–1835. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman E. S., Newball H. H., Demers L. M., Fitzpatrick F. A., Adkinson N. F., Jr Anaphylactic release of thromboxane A2, prostaglandin D2, and prostacyclin from human lung parenchyma. Am Rev Respir Dis. 1981 Oct;124(4):402–406. doi: 10.1164/arrd.1981.124.4.402. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Liberman E., Raz A. Bradykinin and angiotensin II activation of arachidonic acid deacylation and prostaglandin E2 formation in rabbit kidney. Hormone-sensitive versus hormone-insensitive lipid pools of arachidonic acid. J Biol Chem. 1981 Mar 10;256(5):2329–2333. [PubMed] [Google Scholar]
- Sun F. F., Chapman J. P., McGuire J. C. Metabolism of prostaglandin endoperoxide in animal tissues. Prostaglandins. 1977;14(6):1055–1074. doi: 10.1016/0090-6980(77)90285-4. [DOI] [PubMed] [Google Scholar]
- Westcott J. Y., Clay K. L., Murphy R. C. Decomposition of leukotriene C4. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):363–368. doi: 10.1016/0091-6749(84)90131-3. [DOI] [PubMed] [Google Scholar]


