Abstract
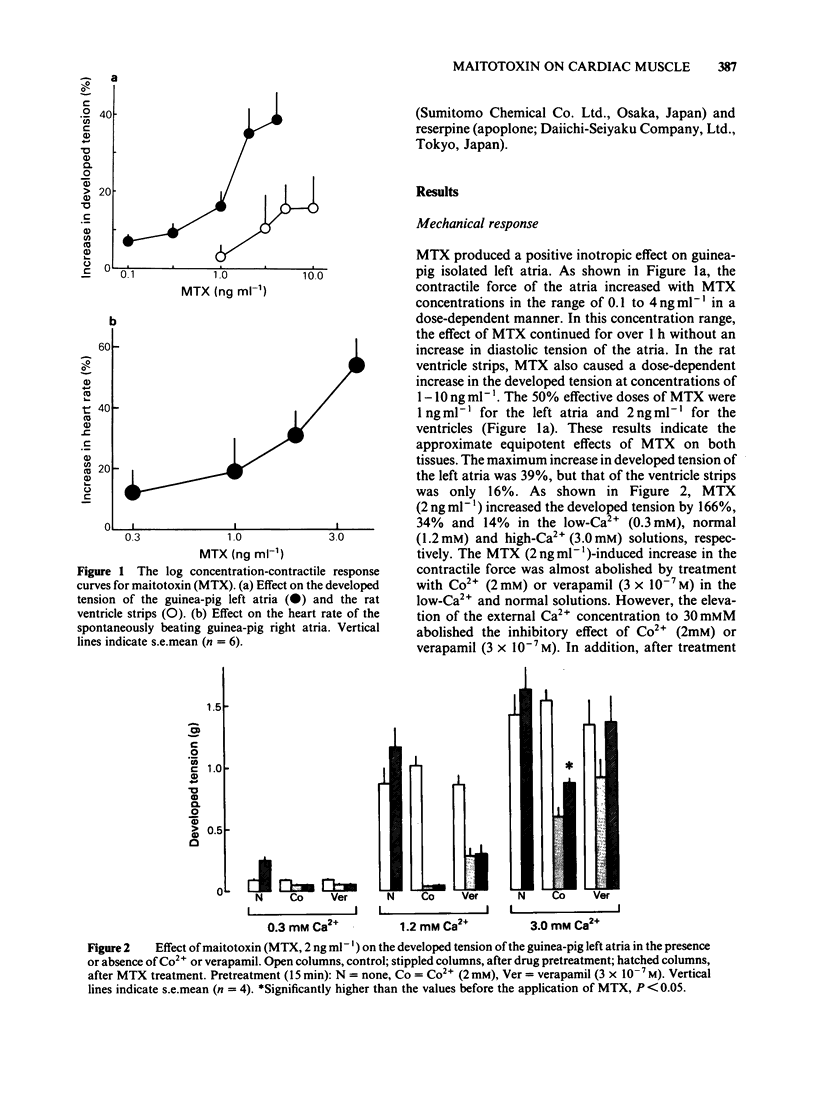
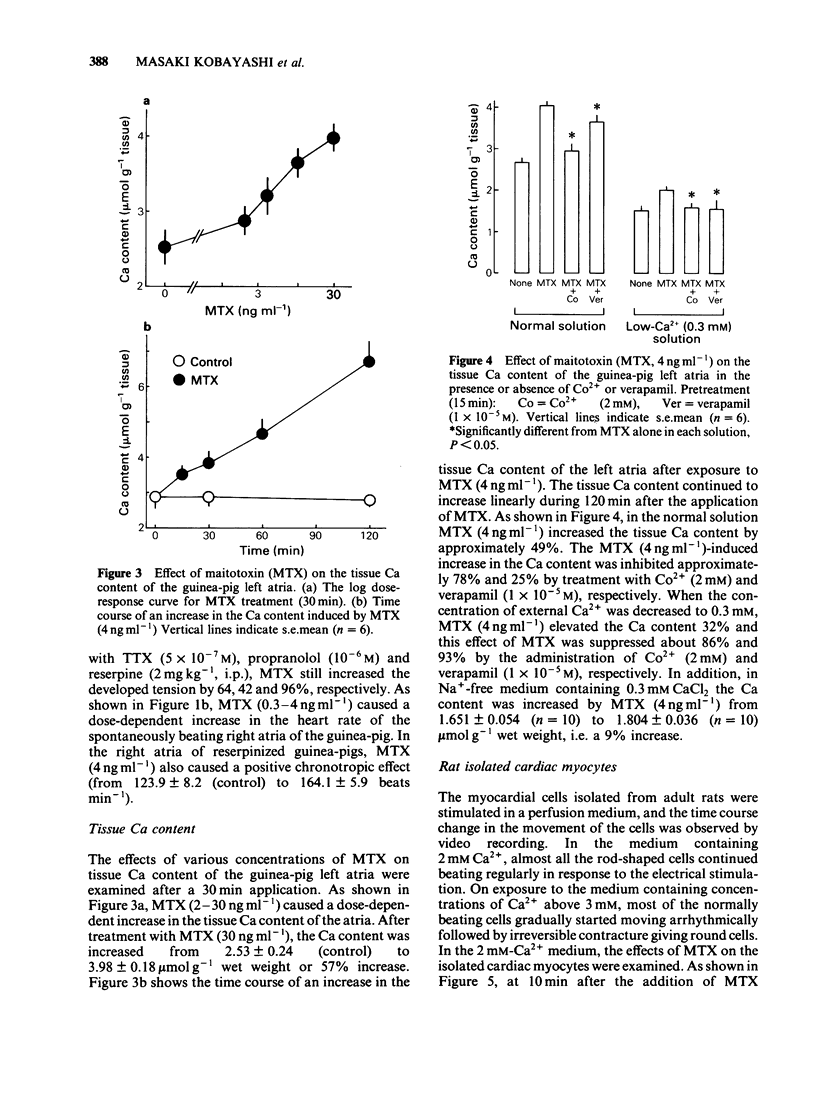
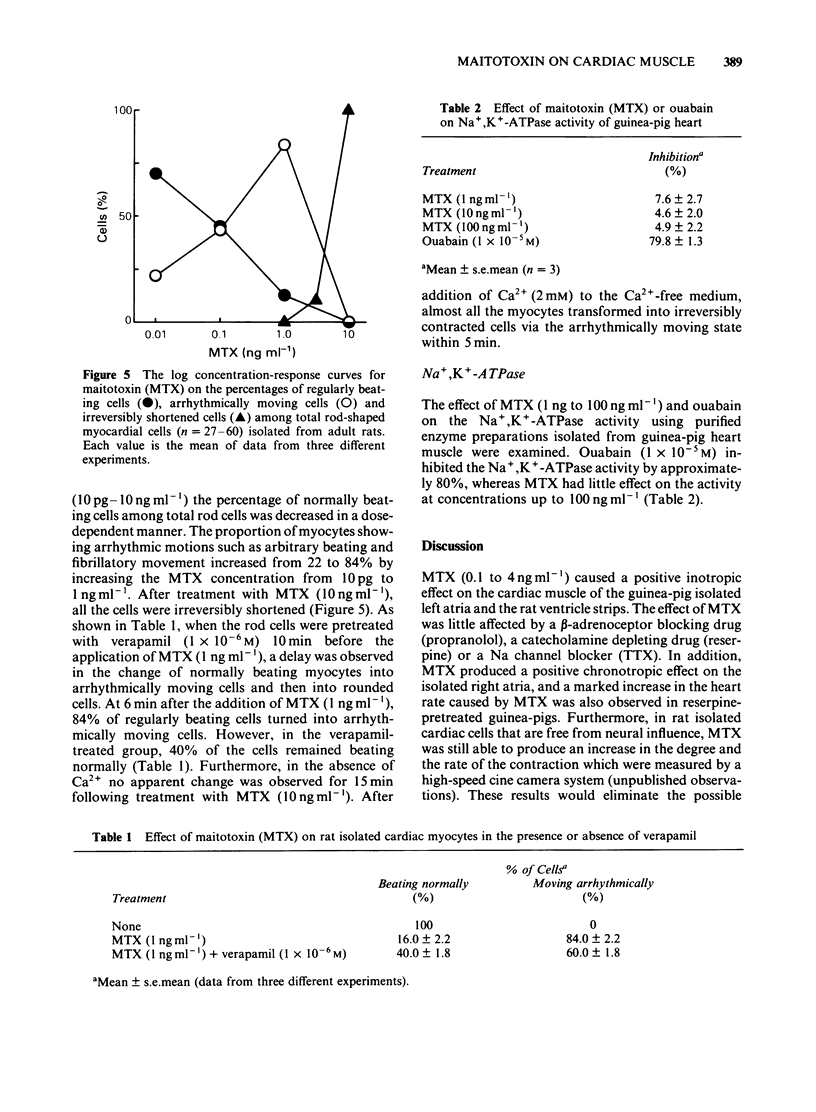
Maitotoxin (MTX), the most potent marine toxin known, produced a dose-dependent positive inotropic effect on guinea-pig isolated left atria and rat ventricle strips at concentrations of 0.1 ng to 4 ng ml-1. MTX (4 ng ml-1) also exhibited a positive chronotropic effect on guinea-pig right atria. The MTX-induced inotropic effect was almost abolished by Co2+ or verapamil, but was little affected by propranolol, reserpine or tetrodotoxin. The tissue Ca content of guinea-pig left atria was increased by MTX (2-30 ng ml-1) in a dose-dependent manner, and this increase was markedly inhibited by Co2+ or verapamil. Furthermore, on the rat isolated cardiac myocytes MTX (0.01-10 ng ml-1) caused an arrhythmogenic effect which was followed by their transformation into irreversibly rounded cells. The effects of MTX on the isolated cells were inhibited by verapamil or Ca2+-free solution. These results suggest that the excitatory effects of MTX on heart muscle are caused by a direct action on the cardiac muscle membrane mainly due to an increase in Ca2+ permeability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W., Mouri T., Nakazato Y. Calcium and stimulus-secretion coupling in the adrenal medulla: contrasting stimulating effects of the ionophores X-537A and A23187 on catecholamine output. J Physiol. 1975 Nov;252(2):363–378. doi: 10.1113/jphysiol.1975.sp011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow J. W., Harding N. G., Powell T. Isolated cardiac myocytes. I. Preparation of adult myocytes and their homology with the intact tissue. Cardiovasc Res. 1981 Sep;15(9):483–514. doi: 10.1093/cvr/15.9.483. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J., Miller D. M., Tindall D. R. Interactions of maitotoxin with voltage-sensitive calcium channels in cultured neuronal cells. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4582–4585. doi: 10.1073/pnas.81.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M., Prat J. C. A calcium ionophore stimulating the secretion of catecholamines from the cat adrenal. J Physiol. 1975 Jan;244(1):253–262. doi: 10.1113/jphysiol.1975.sp010795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D. R., Steinberg M. I., Armstrong W. M. A23187: a calcium ionophore that directly increases cardiac contractility (38704). Proc Soc Exp Biol Med. 1975 Apr;148(4):1141–1145. [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The intrinsic control of myocardial contraction--ionic factors. N Engl J Med. 1971 Nov 4;285(19):1065–1071. doi: 10.1056/NEJM197111042851910. [DOI] [PubMed] [Google Scholar]
- Legrand A. M., Bagnis R. Effects of ciguatoxin and maitotoxin on isolated rat atria and rabbit duodenum. Toxicon. 1984;22(3):471–475. doi: 10.1016/0041-0101(84)90091-6. [DOI] [PubMed] [Google Scholar]
- Legrand A. M., Bagnis R. Effects of highly purified maitotoxin extracted from dinoflagellate Gambierdiscus toxicus on action potential of isolated rat heart. J Mol Cell Cardiol. 1984 Jul;16(7):663–666. doi: 10.1016/s0022-2828(84)80630-6. [DOI] [PubMed] [Google Scholar]
- Miyahara J. T., Akau C. K., Yasumoto T. Effects of ciguatoxin and maitotoxin on the isolated guinea pig atria. Res Commun Chem Pathol Pharmacol. 1979 Jul;25(1):177–180. [PubMed] [Google Scholar]
- Miyakoda G., Nakamura T. Reversible effects of a high-molecular weight sulfhydryl reagent on the contractile activity of myocardial cells isolated from adult rat heart. J Biochem. 1982 Dec;92(6):1833–1843. doi: 10.1093/oxfordjournals.jbchem.a134113. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Ohizumi Y., Washio H., Yasumoto Y. Potent excitatory effect of maitotoxin on Ca channels in the insect skeletal muscle. Pflugers Arch. 1984 Apr;400(4):439–441. doi: 10.1007/BF00587546. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Norton T. R., Ohizumi Y., Shibata S. Excitatory effect of a new polypeptide (anthopleurin-B) from sea anemone on the guinea-pig vas deferens. Br J Pharmacol. 1981 Sep;74(1):23–28. doi: 10.1111/j.1476-5381.1981.tb09951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohizumi Y., Kajiwara A., Yasumoto T. Excitatory effect of the most potent marine toxin, maitotoxin, on the guinea-pig vas deferens. J Pharmacol Exp Ther. 1983 Oct;227(1):199–204. [PubMed] [Google Scholar]
- Ohizumi Y., Shibata S. Mechanism of the excitatory action of palytoxin and N-acetylpalytoxin in the isolated guinea-pig vas deferens. J Pharmacol Exp Ther. 1980 Jul;214(1):209–212. [PubMed] [Google Scholar]
- Ohizumi Y., Shibata S. Nature of anthopleurin-B-induced release of norepinephrine from adrenergic nerves. Am J Physiol. 1982 Nov;243(5):C237–C241. doi: 10.1152/ajpcell.1982.243.5.C237. [DOI] [PubMed] [Google Scholar]
- Ohizumi Y., Shibata S. Possible mechanism of the dual action of the new polypeptide (anthopleurin-B) from sea anemone in the isolated ileum and taenia caeci of the guinea-pig. Br J Pharmacol. 1981 Feb;72(2):239–244. doi: 10.1111/j.1476-5381.1981.tb09119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohizumi Y., Yasumoto T. Contraction and increase in tissue calcium content induced by maitotoxin, the most potent known marine toxin, in intestinal smooth muscle. Br J Pharmacol. 1983 May;79(1):3–5. doi: 10.1111/j.1476-5381.1983.tb10485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts B. J., Schwartz A. Improved purification and partial characterization of (Na+, K+)-ATPase from cardiac muscle. Biochim Biophys Acta. 1975 Aug 20;401(2):184–195. doi: 10.1016/0005-2736(75)90303-x. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Romey G., Abita J. P., Schweitz H., Wunderer G., Lazdunski Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettini G., Koike K., Login I. S., Judd A. M., Cronin M. J., Yasumoto T., MacLeod R. M. Maitotoxin stimulates hormonal release and calcium flux in rat anterior pituitary cells in vitro. Am J Physiol. 1984 Oct;247(4 Pt 1):E520–E525. doi: 10.1152/ajpendo.1984.247.4.E520. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ohizumi Y., Yasumoto T. Maitotoxin, a Ca2+ channel activator candidate. J Biol Chem. 1982 Jul 10;257(13):7287–7289. [PubMed] [Google Scholar]
- Takahashi M., Tatsumi M., Ohizumi Y., Yasumoto T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J Biol Chem. 1983 Sep 25;258(18):10944–10949. [PubMed] [Google Scholar]
- Williamson J. R., Williams R. J., Coll K. E., Thomas A. P. Cytosolic free Ca2+ concentration and intracellular calcium distribution of Ca2+-tolerant isolated heart cells. J Biol Chem. 1983 Nov 25;258(22):13411–13414. [PubMed] [Google Scholar]


