Abstract
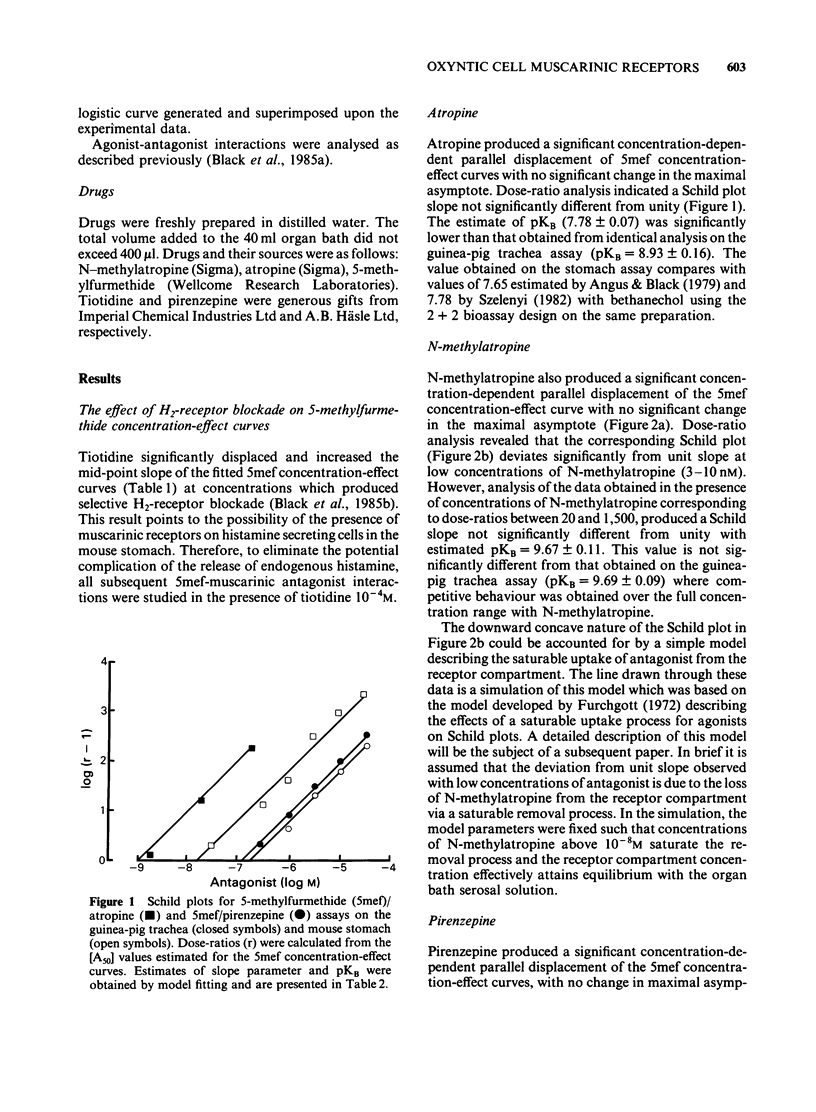
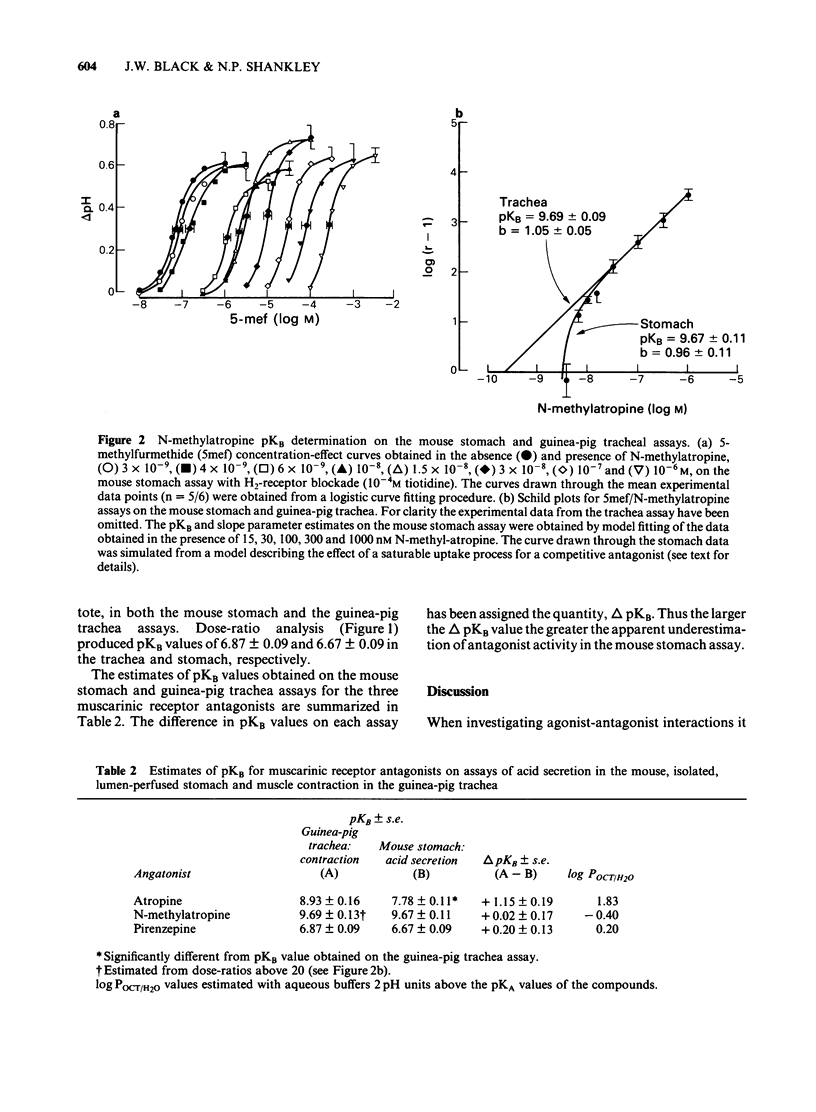
In the light of recent attempts to subclassify muscarinic receptors, agonist-antagonist interactions at muscarinic receptors have been re-examined using improved techniques, on the mouse, isolated, lumen-perfused stomach gastric acid assay. Using 5-methylfurmethide as the muscarinic agonist, the pKB estimated for atropine was significantly lower on the stomach assay (7.78) than on the guinea-pig trachea (8.93). However pKB values for N-methylatropine, the quaternary ammonium derivative of atropine, at concentrations producing dose-ratios above 20 on the stomach assay (pKB = 9.67), and over the full concentration range studied on the trachea (pKB = 9.69) were not significantly different. The deviation from simple competitive behaviour at low dose-ratios with N-methylatropine in the stomach assay is consistent with the effects of a saturable uptake mechanism for quaternary ammonium compounds. The pKB values for pirenzepine on the stomach (6.67) and the trachea (6.87) were not significantly different suggesting that pirenzepine behaves more like N-methylatropine in terms of expressed affinity. We conclude that the oxyntic cell muscarinic receptors are homogeneous with those in the guinea-pig trachea. An initial exploration suggests that there is a relationship between the lipophilicity (log P) of the antagonists and the degree of apparent underestimation of antagonist affinity in the stomach assay. This supports the hypothesis that the underestimation of antagonist affinity is due to the loss of antagonist into the gastric secretion from the receptor compartment. Apparently, relatively selective inhibition of acid secretion, compared to atropine, could be explained without the need to postulate heterogeneity of muscarinic receptor populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Black J. W. Analysis of anomalous pKb values for metiamide and atropine in the isolated stomach of the mouse. Br J Pharmacol. 1979 Sep;67(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Black J. W., Stone M. Estimation of pKB values for histamine H2-receptor antagonists using an in vitro acid secretion assay. Br J Pharmacol. 1980 Mar;68(3):413–423. doi: 10.1111/j.1476-5381.1980.tb14555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Black J. W. The interaction of choline esters, vagal stimulation and H2-receptor blockade on acid secretion in vitro. Eur J Pharmacol. 1982 May 21;80(2-3):217–224. doi: 10.1016/0014-2999(82)90057-7. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Chan M. The effects of pH on the affinity of pirenzepine for muscarinic receptors in the guinea-pig ileum and rat fundus strip. Br J Pharmacol. 1982 Nov;77(3):559–563. doi: 10.1111/j.1476-5381.1982.tb09331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br J Pharmacol. 1985 Nov;86(3):581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Pharmacological analysis of the pentagastrin-tiotidine interaction in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):589–599. doi: 10.1111/j.1476-5381.1985.tb08935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Shankley N. P. Pharmacological analysis of the muscarinic receptors involved when McN-A 343 stimulates acid secretion in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):609–617. doi: 10.1111/j.1476-5381.1985.tb08937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Shankley N. P. The isolated stomach preparation of the mouse: a physiological unit for pharmacological analysis. Br J Pharmacol. 1985 Nov;86(3):571–579. doi: 10.1111/j.1476-5381.1985.tb08933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson J., Mackay D. The zig-zag tracheal strip. J Pharm Pharmacol. 1979 Nov;31(11):798–798. doi: 10.1111/j.2042-7158.1979.tb13666.x. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Ruifrok P. G. Uptake of quaternary ammonium compounds into rat intestinal brush border membrane vesicles. Biochem Pharmacol. 1981 Oct 1;30(19):2637–2641. doi: 10.1016/0006-2952(81)90531-1. [DOI] [PubMed] [Google Scholar]
- Ruifrok P. G. Uptake of quaternary ammonium compounds into rat liver plasma membrane vesicles. Biochem Pharmacol. 1982 Apr 1;31(7):1431–1435. doi: 10.1016/0006-2952(82)90039-9. [DOI] [PubMed] [Google Scholar]
- Szelenyi I. Does pirenzepine distinguish between 'subtypes' of muscarinic receptors? Br J Pharmacol. 1982 Dec;77(4):567–569. doi: 10.1111/j.1476-5381.1982.tb09332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


