Abstract
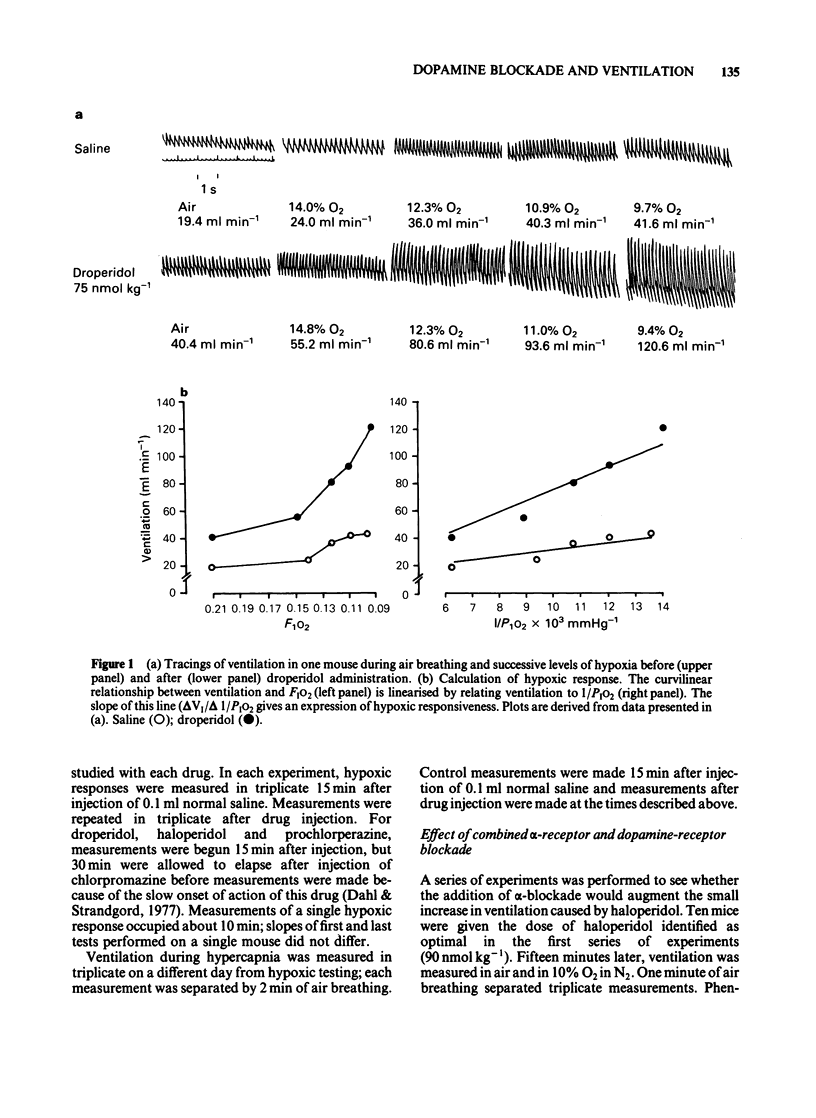
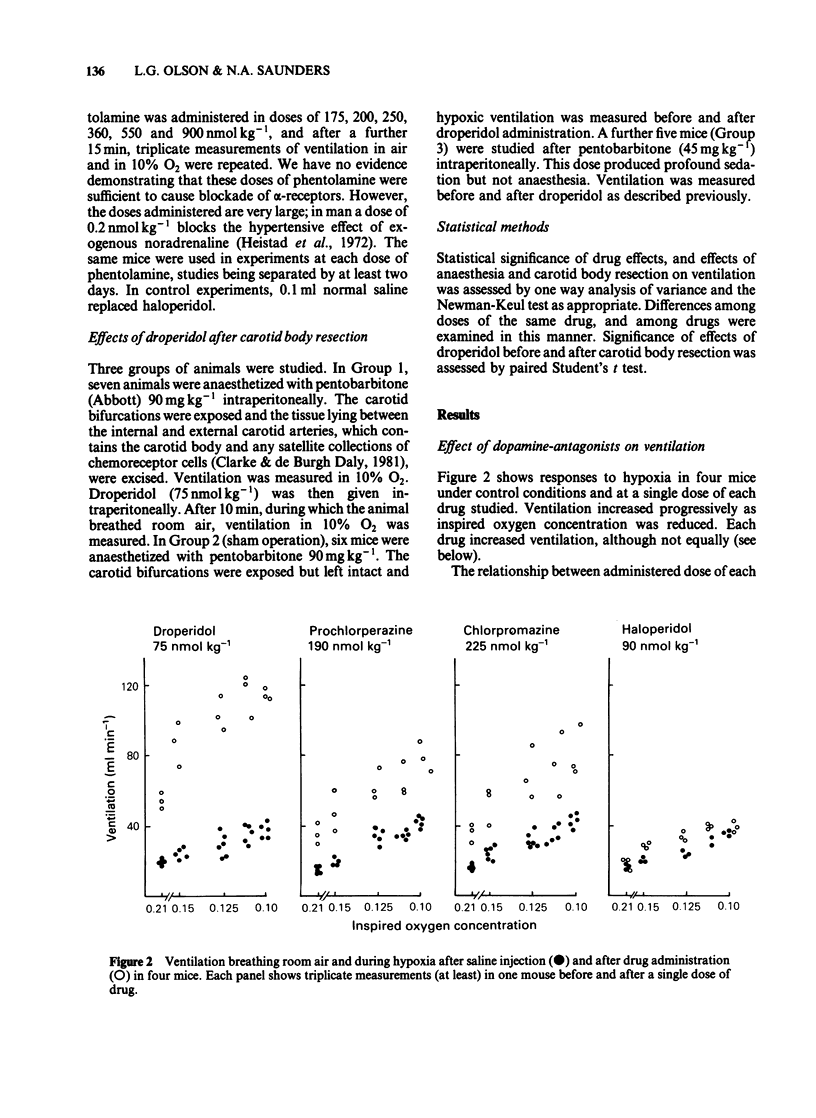
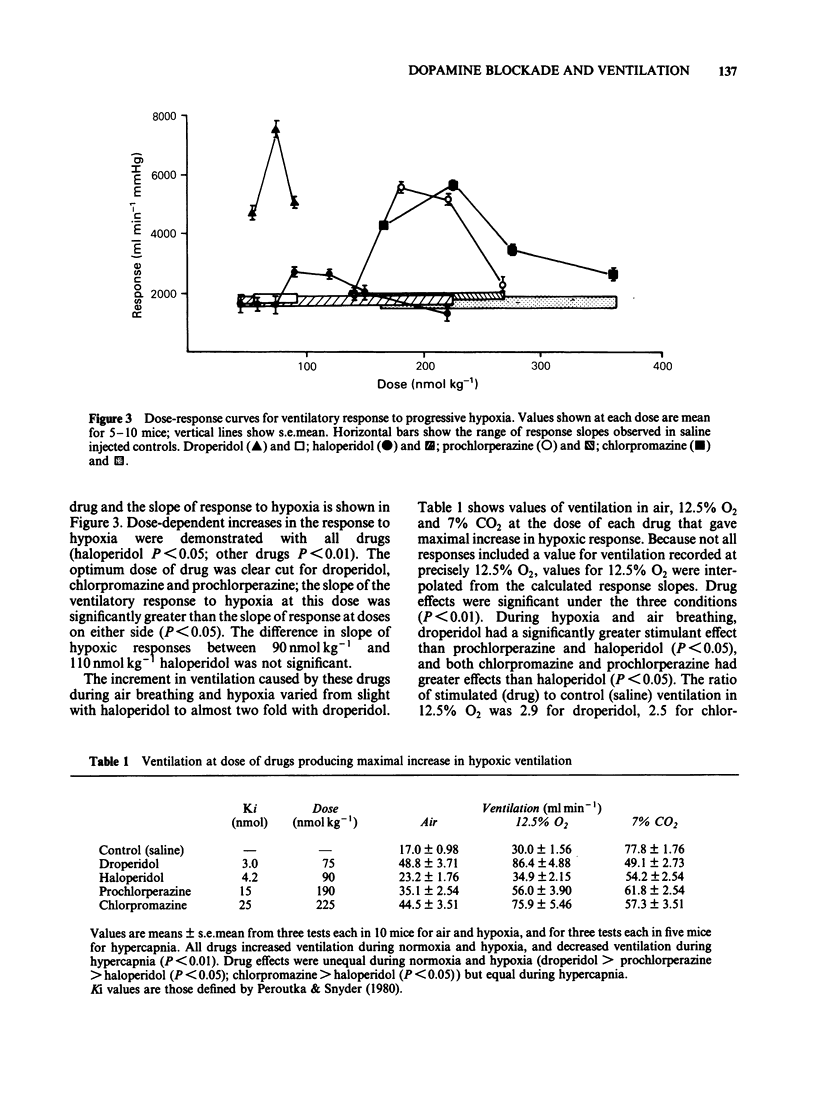
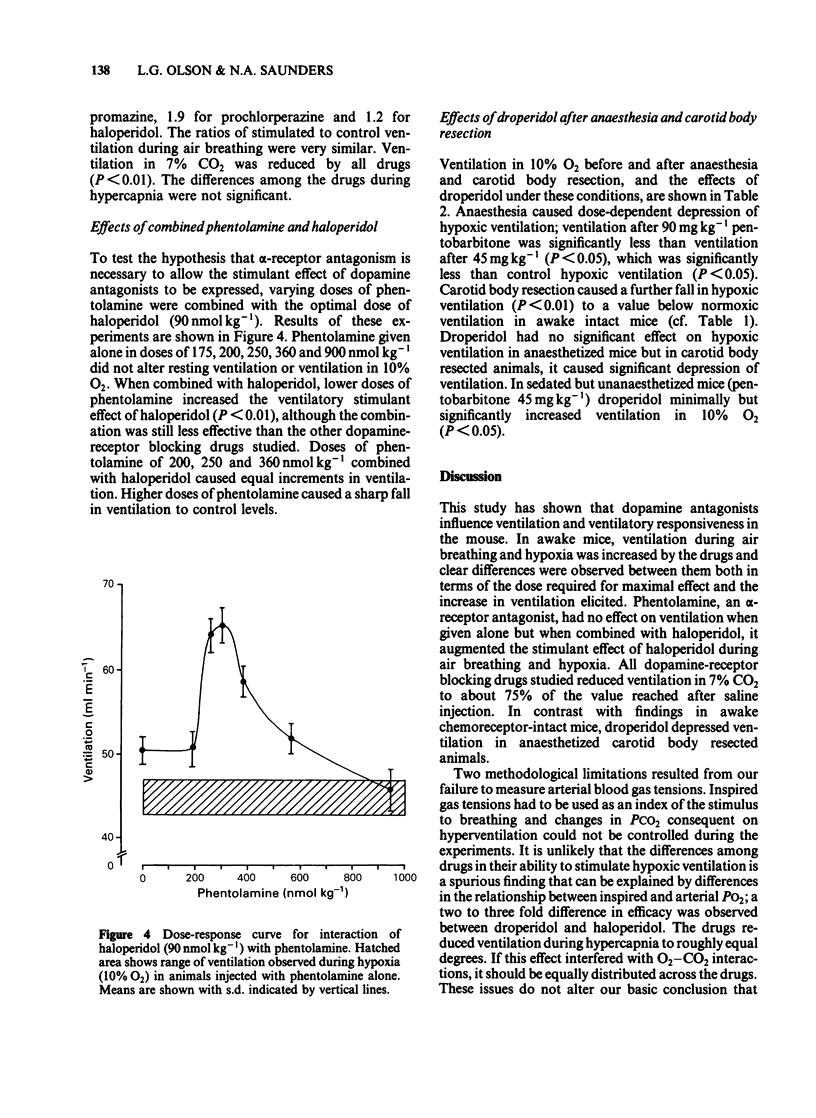
Ventilation was measured by a plethysmographic method in awake mice before and after intraperitoneal injection of neuroleptic drugs to test the hypothesis that dopaminergic mechanisms modulate control of breathing in this species. Dose-dependent augmentation of ventilation at rest and during hypoxia, and reduced ventilation during hypercapnia was demonstrated for haloperidol, droperidol, prochlorperazine and chlorpromazine (P less than 0.05 or less for each drug). Doses of drugs causing maximal increase of the ventilatory response to hypoxia were linearly related (r = 0.98, P less than 0.001) to in vitro affinity of the drugs for dopamine receptors. Despite presumed equal dopamine-receptor blockade, the drugs had unequal effects on the ventilatory response to hypoxia. Droperidol augmented hypoxic ventilation to 290% of the control value, chlorpromazine to 250% control, prochlorperazine to 190% control and haloperidol to 120% control. These differences in efficacy were in the same order as the affinities of the drugs for alpha-adrenoceptors. The effect of combined haloperidol (90 nmol kg-1) and varying doses of phentolamine (175-900 nmol kg-1) was assessed to test the hypothesis that alpha-antagonism was a factor in determining the increase in ventilation following dopamine blockade. Phentolamine caused dose-dependent augmentation of the ventilatory effects of haloperidol (P less than 0.01) but had no ventilatory effect when given alone. Carotid body resection in anaesthetized mice abolished the stimulation of hypoxic ventilation caused by droperidol. It is concluded that dopaminergic mechanisms in the carotid body modulate ventilatory control in the awake mouse. The drugs most effective in augmenting hypoxic ventilation are those that block both dopamine and alpha-adrenoceptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff M. J., Jaffe R. A., Sampson S. R., Vidruk E. H. Effects of droperidol on activity of carotid body chemoreceptors in cat. Br J Pharmacol. 1978 Jun;63(2):245–250. doi: 10.1111/j.1476-5381.1978.tb09753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge C. W., Heistad D. D. Effect of haloperidol on ventilatory responses to dopamine in man. J Pharmacol Exp Ther. 1980 Apr;213(1):13–17. [PubMed] [Google Scholar]
- Bartlett D., Jr, Tenney S. M. Control of breathing in experimental anemia. Respir Physiol. 1970 Oct;10(3):384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Belmonte C., Eyzaguirre C. Efferent influences on carotid body chemoreceptors. J Neurophysiol. 1974 Nov;37(6):1131–1143. doi: 10.1152/jn.1974.37.6.1131. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Bradley G. W., Purves M. J. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol. 1970 May;208(1):99–120. doi: 10.1113/jphysiol.1970.sp009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas H., Zapata P. Dopamine-induced ventilatory depression in the rat, mediated by carotid nerve afferents. Neurosci Lett. 1981 Jun 12;24(1):29–33. doi: 10.1016/0304-3940(81)90354-2. [DOI] [PubMed] [Google Scholar]
- Clarke J. A., de Burgh Daly M. A comparative study of the distribution of carotid body type-I cells and periadventitial type-I cells in the carotid bifurcation regions of the rabbit, rat, guinea-pig and mouse. Cell Tissue Res. 1981;220(4):753–772. doi: 10.1007/BF00210459. [DOI] [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976 Apr 30;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- DRORBAUGH J. E., FENN W. O. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955 Jul;16(1):81–87. [PubMed] [Google Scholar]
- Dahl S. G., Strandjord R. E. Pharmacokinetics of chlorpromazine after single and chronic dosage. Clin Pharmacol Ther. 1977 Apr;21(4):437–448. doi: 10.1002/cpt1977214437. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol. 1978 Jun;279:425–436. doi: 10.1113/jphysiol.1978.sp012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Zapata P. The release of acetylcholine from carotid body tissues. Further study on the effects of acetylcholine and cholinergic blocking agents on the chemosensory discharge. J Physiol. 1968 Apr;195(3):589–607. doi: 10.1113/jphysiol.1968.sp008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I., Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978 Sep;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad D. D., Wheeler R. C., Mark A. L., Schmid P. G., Abboud F. M. Effects of adrenergic stimulation on ventilation in man. J Clin Invest. 1972 Jun;51(6):1469–1475. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of adrenoceptor stimulating and blocking agents on carotid body chemosensory inhibition. J Physiol. 1978 Jan;274:501–509. doi: 10.1113/jphysiol.1978.sp012163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol. 1978 Jan;274:487–499. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D., Breese G. R., Mueller R. A. Dopaminergic interaction with the respiratory control system in the rat. Eur J Pharmacol. 1979 Feb 15;54(1-2):153–159. doi: 10.1016/0014-2999(79)90417-5. [DOI] [PubMed] [Google Scholar]
- Mir A. K., McQueen D. S., Pallot D. J., Nahorski S. R. Direct biochemical and neuropharmacological identification of dopamine D2-receptors in the rabbit carotid body. Brain Res. 1984 Jan 23;291(2):273–283. doi: 10.1016/0006-8993(84)91259-9. [DOI] [PubMed] [Google Scholar]
- Olson L. G., Hensley M. J., Saunders N. A. Augmentation of ventilatory response to asphyxia by prochlorperazine in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Sep;53(3):637–643. doi: 10.1152/jappl.1982.53.3.637. [DOI] [PubMed] [Google Scholar]
- Olson L. G., Hensley M. J., Saunders N. A. Ventilatory responsiveness to hypercapnic hypoxia during dopamine infusion in humans. Am Rev Respir Dis. 1982 Nov;126(5):783–787. doi: 10.1164/arrd.1982.126.5.783. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Synder S. H. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980 Dec;137(12):1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- Sampson S. R. Mechanism of efferent inhibition of carotid body chemoreceptors in the cat. Brain Res. 1972 Oct 13;45(1):266–270. doi: 10.1016/0006-8993(72)90236-3. [DOI] [PubMed] [Google Scholar]
- Smatresk N. J., Pokorski M., Lahiri S. Opposing effects of dopamine receptor blockade on ventilation and carotid chemoreceptor activity. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jun;54(6):1567–1573. doi: 10.1152/jappl.1983.54.6.1567. [DOI] [PubMed] [Google Scholar]
- Zapata P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J Physiol. 1975 Jan;244(1):235–251. doi: 10.1113/jphysiol.1975.sp010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata P., Zuazo A. Respiratory effects of dopamine-induced inhibition of chemosensory inflow. Respir Physiol. 1980 Apr;40(1):79–92. doi: 10.1016/0034-5687(80)90006-7. [DOI] [PubMed] [Google Scholar]
- Zapata P., Zuazo A. Reversal of respiratory responses to dopamine after dopamine antagonists. Respir Physiol. 1982 Feb;47(2):239–255. doi: 10.1016/0034-5687(82)90114-1. [DOI] [PubMed] [Google Scholar]


