Abstract
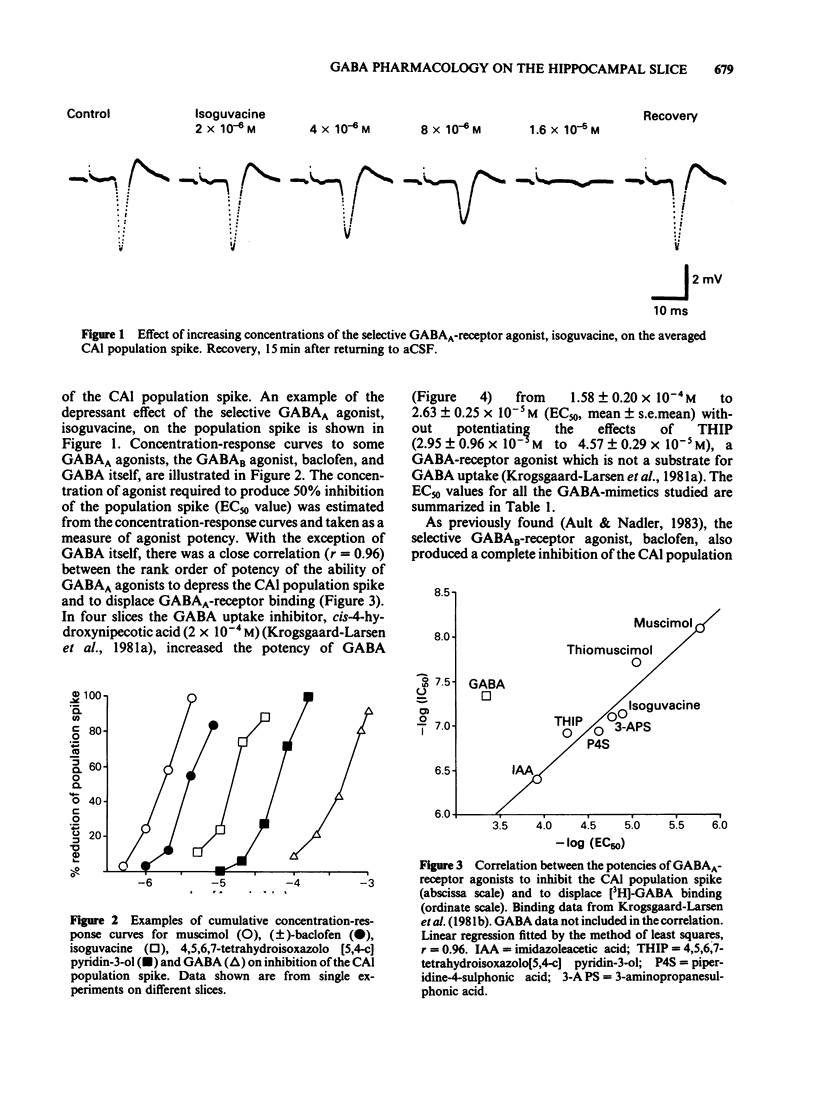
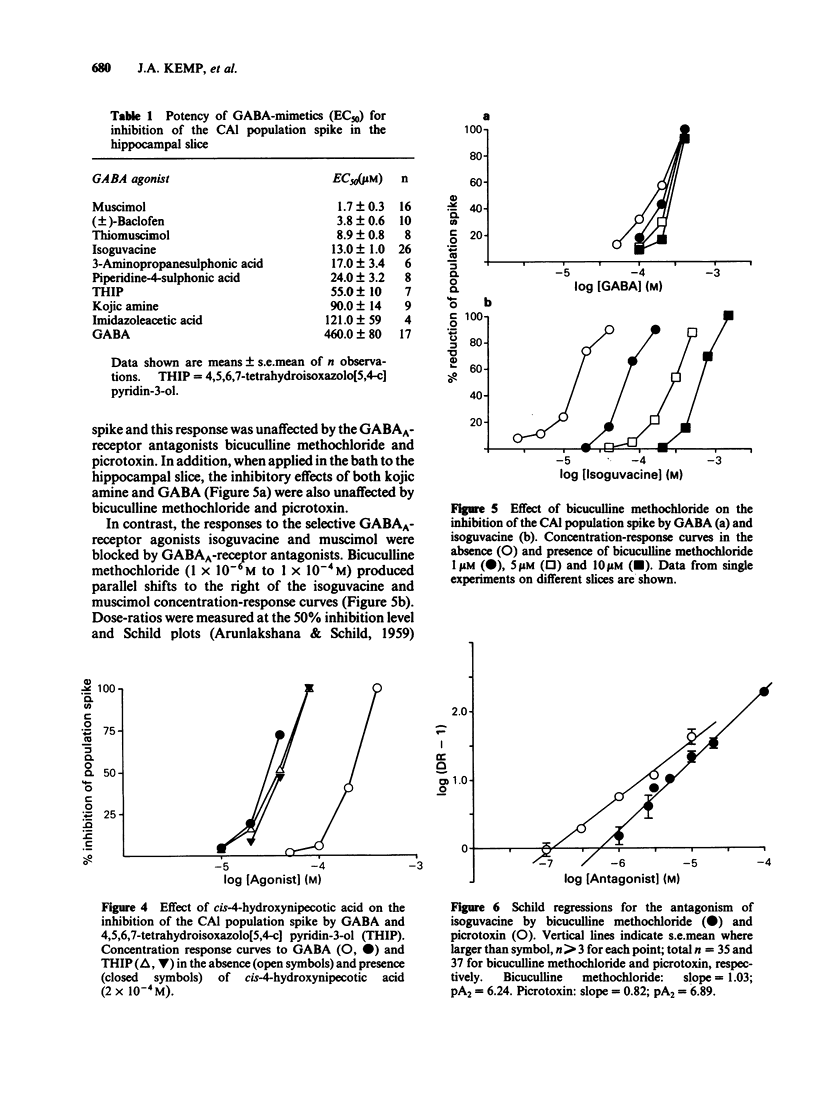
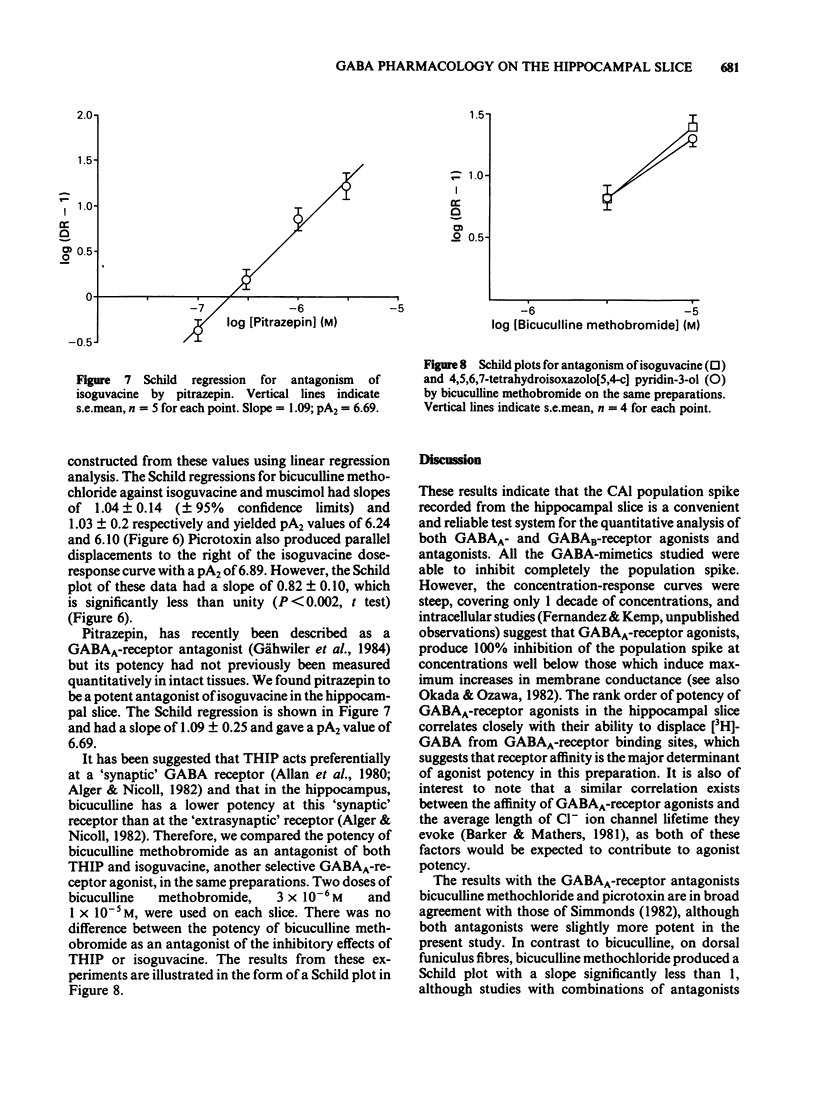
CA1 population spikes recorded in the rat hippocampal slice were used to assess quantitatively the potencies of GABA-receptor agonists and antagonists on mammalian CNS neurones. Apart from GABA itself, GABA A-receptor agonists inhibited the CA1 population spikes with potencies that correlated closely (r = 0.96) with their ability to displace [3H]-GABA from GABAA-binding sites. The low potency of GABA in this preparation was attributed to the action of uptake processes as the GABA uptake inhibitor, cis-4-hydroxynipecotic acid (2 X 10(-4) M), produced an approximate 6 fold increase in the potency of GABA whilst having no effect on the potency of 4,5,6,7-tetrahydroisoxazolo [5,4-c] pyridin-3-ol (THIP), a GABAA-receptor agonist which is not a substrate for the GABA uptake system. The inhibitory effects of the selective GABAA-receptor agonists isoguvacine and muscimol were antagonized by bicuculline methochloride, which shifted the dose-response curves to the right in a parallel manner. The Schild plots for bicuculline methochloride against isoguvacine and muscimol had slopes of 1 and gave pA2 values of 6.24 and 6.10, respectively. Picrotoxin also antagonized the inhibitory effects of isoguvacine and produced parallel shifts to the right of the dose-response curve. However, the Schild plot for picrotoxin had a slope significantly less than unity (0.82) and gave a pA2 value of 6.89. The novel GABAA-receptor antagonist, pitrazepin, antagonized the inhibitory effects of isoguvacine in an apparently competitive manner. The Schild plot had a slope of 1 and gave a pA2 of 6.69.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan R. D., Evans R. H., Johnston G. A. gamma-Aminobutyric acid agonists: an in vitro comparison between depression of spinal synaptic activity and depolarization of spinal root fibres in the rat. Br J Pharmacol. 1980 Dec;70(4):609–615. doi: 10.1111/j.1476-5381.1980.tb09779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982 Nov;223(2):291–297. [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Effects of baclofen on synaptically-induced cell firing in the rat hippocampal slice. Br J Pharmacol. 1983 Sep;80(1):211–219. doi: 10.1111/j.1476-5381.1983.tb11068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Mathers D. A. GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science. 1981 Apr 17;212(4492):358–361. doi: 10.1126/science.6259733. [DOI] [PubMed] [Google Scholar]
- Bartholini G. GABA receptor agonists: pharmacological spectrum and therapeutic actions. Med Res Rev. 1985 Jan-Mar;5(1):55–75. doi: 10.1002/med.2610050103. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Doble A., Hill D. R., Hudson A. L., Shaw J. S., Turnbull M. J., Warrington R. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol. 1981 Apr 24;71(1):53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Inhibition of GABA uptake potentiates the conductance increase produced by GABA-mimetic compounds on single neurones in isolated olfactory cortex slices of the guinea-pig. Br J Pharmacol. 1984 Sep;83(1):195–202. doi: 10.1111/j.1476-5381.1984.tb10135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Johnston G. A. The uptake of GABA into rat spinal roots. J Neurochem. 1974 Jun;22(6):931–935. doi: 10.1111/j.1471-4159.1974.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T., Mueller A., Basile A. The use of brain slices in central nervous system pharmacology. Fed Proc. 1983 Sep;42(12):2891–2898. [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Maurer R., Wüthrich H. J. Pitrazepin, a novel GABAA antagonist. Neurosci Lett. 1984 Apr 6;45(3):311–316. doi: 10.1016/0304-3940(84)90244-1. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R. GABAB receptor modulation of adenylate cyclase activity in rat brain slices. Br J Pharmacol. 1985 Jan;84(1):249–257. [PMC free article] [PubMed] [Google Scholar]
- Karbon E. W., Duman R. S., Enna S. J. GABAB receptors and norepinephrine-stimulated cAMP production in rat brain cortex. Brain Res. 1984 Jul 23;306(1-2):327–332. doi: 10.1016/0006-8993(84)90382-2. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Snowman A., Lummis S. C., Olsen R. W. Characterization of the binding of the GABA agonist [3H]piperidine-4-sulphonic acid to bovine brain synaptic membranes. J Neurochem. 1981 Aug;37(2):401–409. doi: 10.1111/j.1471-4159.1981.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Baudry M., Fagni L., Lynch G. The blocking action of baclofen on excitatory transmission in the rat hippocampal slice. J Neurosci. 1982 Jun;2(6):698–703. doi: 10.1523/JNEUROSCI.02-06-00698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. [3H]bicuculline methochloride binding to low-affinity gamma-aminobutyric acid receptor sites. J Neurochem. 1983 Dec;41(6):1653–1663. doi: 10.1111/j.1471-4159.1983.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978 Jan 27;140(2):315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Classification of some GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur J Pharmacol. 1982 Jun 4;80(4):347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Distinction between the effects of barbiturates, benzodiazepines and phenytoin on responses to gamma-aminobutyric acid receptor activation and antagonism by bicuculline and picrotoxin. Br J Pharmacol. 1981 Jul;73(3):739–747. doi: 10.1111/j.1476-5381.1981.tb16810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M. A. Presynaptic actions of gamma-aminobutyric acid and some antagonists in a slice preparation of cuneate nucleus. Br J Pharmacol. 1978 Jul;63(3):495–502. doi: 10.1111/j.1476-5381.1978.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Freund T. F., Hodgson A. J., Somogyi J., Beroukas D., Chubb I. W. Identified axo-axonic cells are immunoreactive for GABA in the hippocampus and visual cortex of the cat. Brain Res. 1985 Apr 15;332(1):143–149. doi: 10.1016/0006-8993(85)90397-x. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Smith A. D., Nunzi M. G., Gorio A., Takagi H., Wu J. Y. Glutamate decarboxylase immunoreactivity in the hippocampus of the cat: distribution of immunoreactive synaptic terminals with special reference to the axon initial segment of pyramidal neurons. J Neurosci. 1983 Jul;3(7):1450–1468. doi: 10.1523/JNEUROSCI.03-07-01450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. gamma-aminobutyric acid B receptors are negatively coupled to adenylate cyclase in brain, and in the cerebellum these receptors may be associated with granule cells. Mol Pharmacol. 1984 Jan;25(1):24–28. [PubMed] [Google Scholar]
- Yarbrough G. G., Williams M., Haubrich D. R. The neuropharmacology of a novel gamma-aminobutyric acid analog, kojic amine. Arch Int Pharmacodyn Ther. 1979 Oct;241(2):266–279. [PubMed] [Google Scholar]


