Abstract
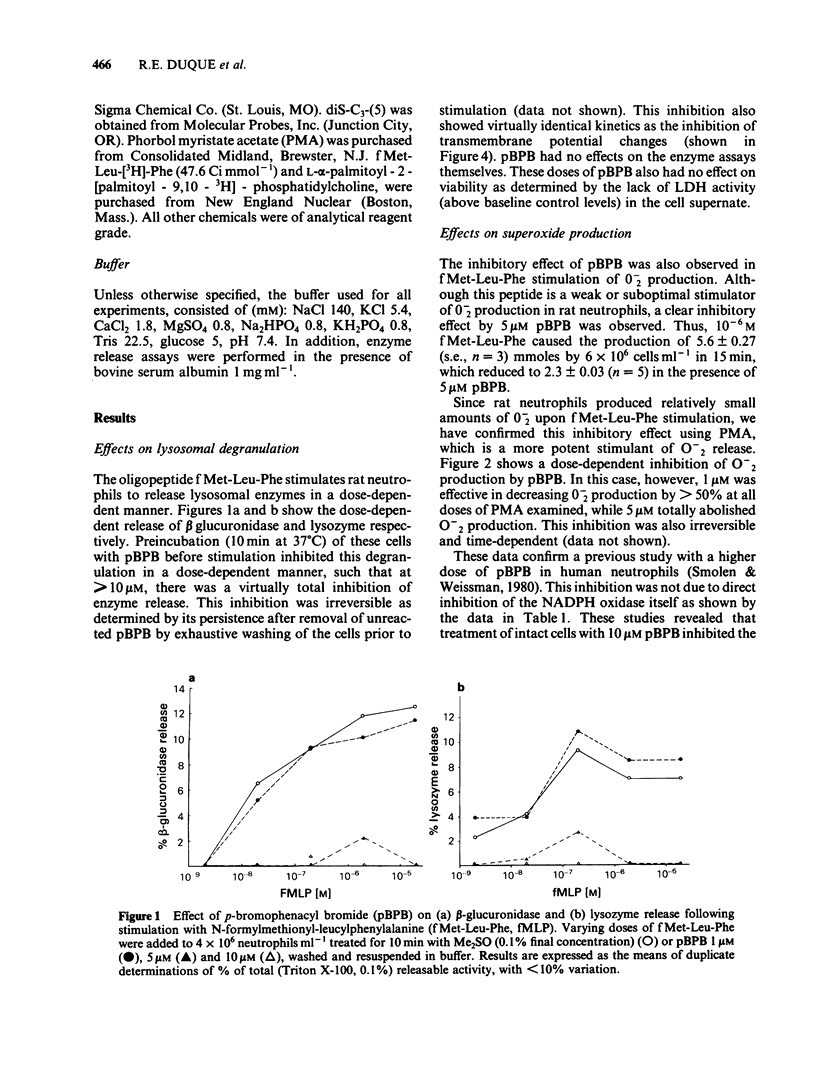
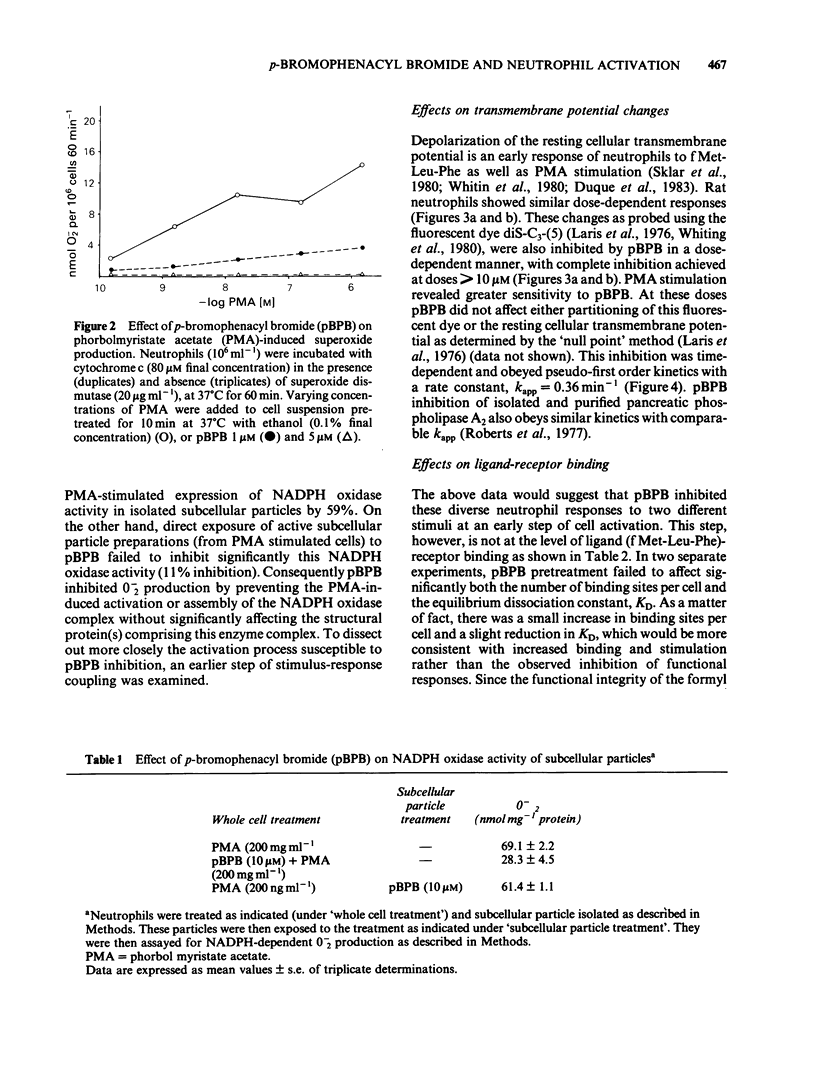
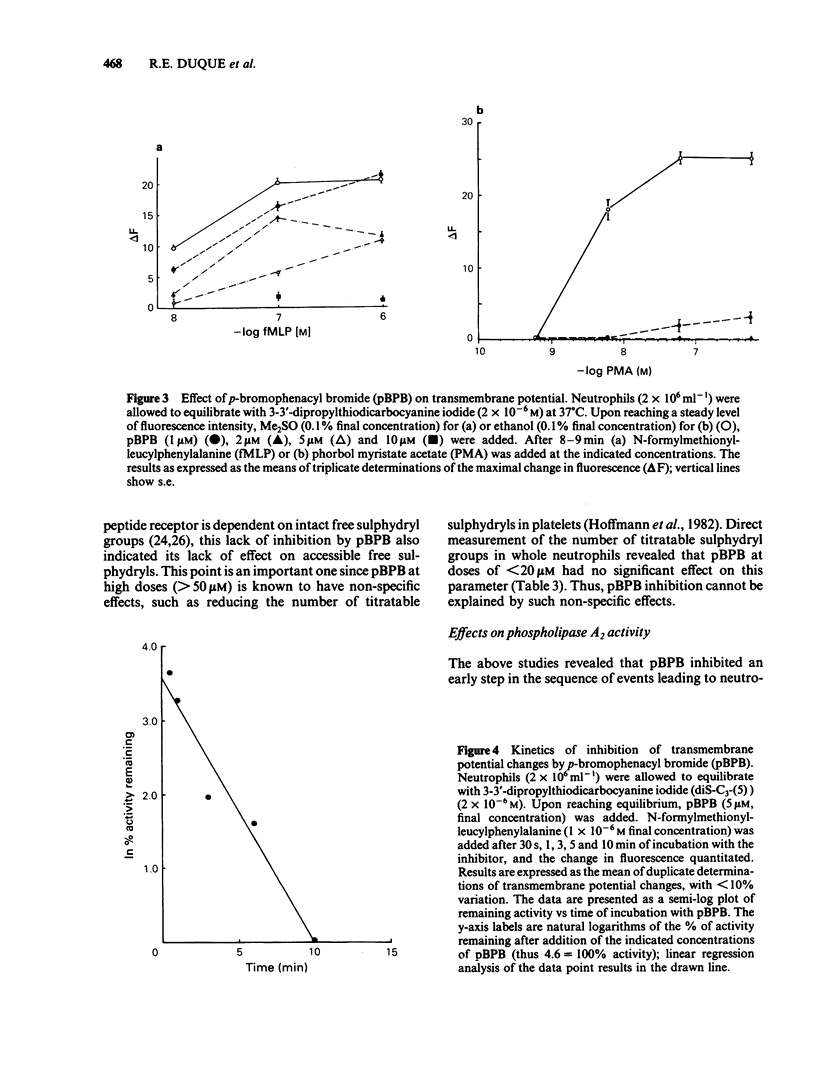
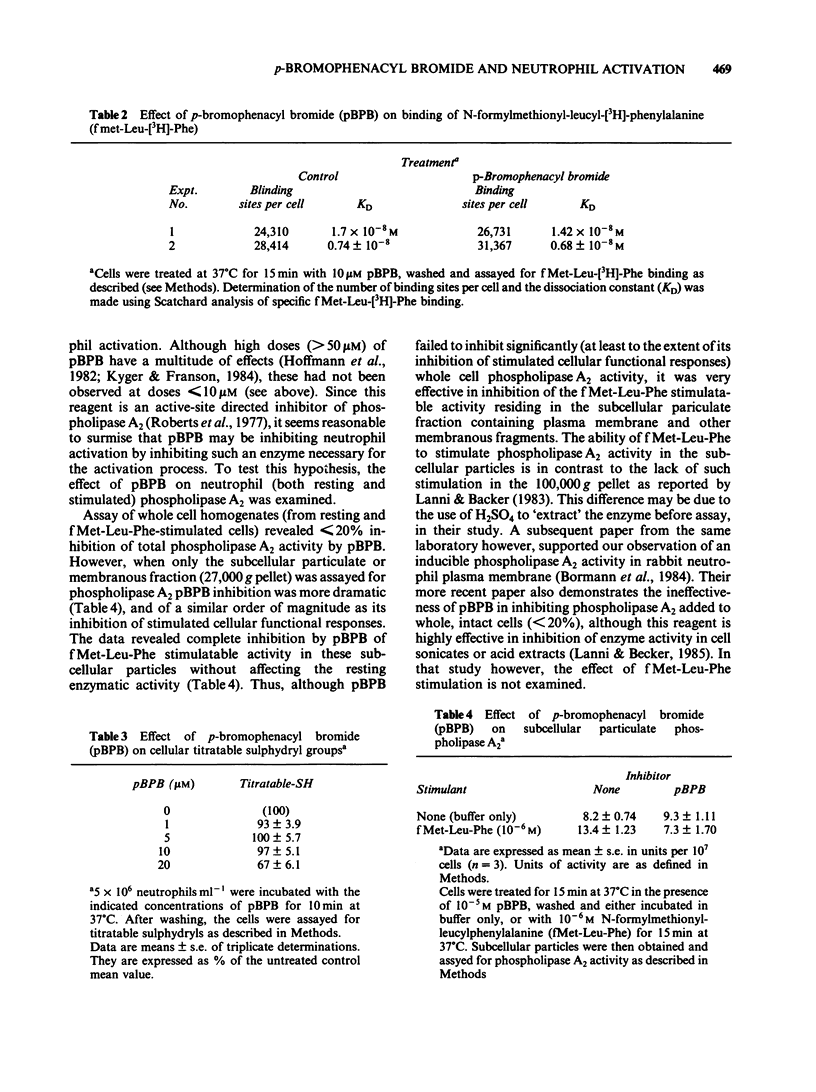
In an effort to elucidate the nature of the inhibitory effects of p-bromophenacyl bromide (pBPB) on neutrophil stimulation, we have examined its effects on several stages of stimulus-response coupling. Pretreatment of rat neutrophils with pBPB resulted in a dose- and time-dependent irreversible inhibition of both N-formylmethionyl-leucylphenylalanine (fMet-Leu-Phe)-induced lysosomal enzyme release and change in transmembrane potential. Inhibition of the biological responses to the chemotactic peptide fMet-Leu-Phe was not due to receptor inactivation since fMet-Leu-[3H]-Phe binding to the formyl peptide receptor was not significantly altered by pBPB pretreatment. Inhibition by pBPB of phorbol myristate acetate (PMA)-induced changes in transmembrane potential and the generation of superoxide (0-2) was also observed. pBPB treatment appeared to inhibit activation of the NADPH oxidase without a direct effect on the oxidase itself. This inhibitory effect was not accompanied by cell death or decrease in cellular titratable sulphydryl groups (at least at doses less than 20 microM). There was, however, significant inhibition of a membranous fraction of fMet-Leu-Phe-induced phospholipase A2 activity by pretreatment with 10 microM pBPB, although total cellular phospholipase A2 was only minimally (less than 20% inhibition) affected. These data would indicate that pBPB inhibits an early event associated with stimulus-response coupling in rat polymorphonuclear leukocytes (i.e. change in transmembrane potential). The inhibitory effects of pBPB may be secondary to the inhibition of a critical membranous fraction of cell bound phospholipase A2 activity or its activation, necessary for the initiation of cell activation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Matter L., Douglas S. D. Effects of acid proteinase inhibitors on human neutrophil chemotaxis and lysosomal enzyme release. II. Bromphenacyl bromide and 1,2-epoxy-3-(p-nitrophenoxy)propane. Clin Immunol Immunopathol. 1983 Feb;26(2):213–222. doi: 10.1016/0090-1229(83)90139-3. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Becker E. L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J Exp Med. 1972 Feb 1;135(2):376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann B. J., Huang C. K., Mackin W. M., Becker E. L. Receptor-mediated activation of a phospholipase A2 in rabbit neutrophil plasma membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):767–770. doi: 10.1073/pnas.81.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAKIN H., ORD M. G., STOCKEN L. A. 'GLUCOSE 6-PHOSPHATE-DEHYDROGENASE' ACTIVITY AND THIOL CONTENT OF THYMUS NUCLEI FROM CONTROL AND X-IRRADIATED RATS. Biochem J. 1963 Nov;89:296–304. doi: 10.1042/bj0890296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen A., Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975 Dec 25;250(24):9342–9347. [PubMed] [Google Scholar]
- Duque R. E., Phan S. H., Sulavik M. C., Ward P. A. Inhibition by tosyl-L-phenylalanyl chloromethyl ketone of membrane potential changes in rat neutrophils. Correlation with the inhibition of biological activity. J Biol Chem. 1983 Jul 10;258(13):8123–8128. [PubMed] [Google Scholar]
- Franson R., Beckerdite S., Wang P., Waite M., Elsbach P. Some properties of phospholipases of alveolar macrophages. Biochim Biophys Acta. 1973 Feb 14;296(2):365–373. doi: 10.1016/0005-2760(73)90094-5. [DOI] [PubMed] [Google Scholar]
- Franson R., Patriarca P., Elsbach P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res. 1974 Jul;15(4):380–388. [PubMed] [Google Scholar]
- Goetzl E. J. Mediators of immediate hypersensitivity derived from arachidonic acid. N Engl J Med. 1980 Oct 2;303(14):822–825. doi: 10.1056/NEJM198010023031421. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. Evidence for an active carboxyl group in pepsin. J Biol Chem. 1966 Aug 10;241(15):3638–3639. [PubMed] [Google Scholar]
- Hofmann S. L., Prescott S. M., Majerus P. W. The effects of mepacrine and p-bromophenacyl bromide on arachidonic acid release in human platelets. Arch Biochem Biophys. 1982 Apr 15;215(1):237–244. doi: 10.1016/0003-9861(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Egan R. W. Prostaglandins, arachidonic acid, and inflammation. Science. 1980 Nov 28;210(4473):978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- Kyger E. M., Franson R. C. Nonspecific inhibition of enzymes by p-bromophenacyl bromide. Inhibition of human platelet phospholipase C and modification of sulfhydryl groups. Biochim Biophys Acta. 1984 Jun 6;794(1):96–103. doi: 10.1016/0005-2760(84)90302-3. [DOI] [PubMed] [Google Scholar]
- Lanni C., Becker E. L. Inhibition of neutrophil phospholipase A2 by p-bromophenylacyl bromide, nordihydroguaiaretic acid, 5,8,11,14-eicosatetraynoic acid and quercetin. Int Arch Allergy Appl Immunol. 1985;76(3):214–217. doi: 10.1159/000233694. [DOI] [PubMed] [Google Scholar]
- Lanni C., Becker E. L. Release of phospholipase A2 activity from rabbit peritoneal neutrophils by f-Met-Leu-Phe. Am J Pathol. 1983 Oct;113(1):90–94. [PMC free article] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A., Johnstone R. M. Monitoring membrane potentials in Ehrlich ascites tumor cells by means of a fluorescent dye. Biochim Biophys Acta. 1976 Jun 17;436(2):475–488. doi: 10.1016/0005-2736(76)90209-1. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Fantone J. C., Freer R. J., Ward P. A. Characterization of the rat neutrophil formyl peptide chemotaxis receptor. Am J Pathol. 1983 Jun;111(3):273–281. [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S. Further characterization of NADPH oxidase activity of human polymorphonuclear leukocytes. J Clin Invest. 1976 Oct;58(4):774–780. doi: 10.1172/JCI108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. Detergent solubilization of the formyl peptide chemotactic receptor. Strategy based on covalent affinity labeling. J Biol Chem. 1981 Sep 10;256(17):9295–9299. [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- Schiffmann E., Aswanikumar S., Venkatasubramanian K., Corcoran B. A., Pert C. B., Brown J., Gross E., Day A. R., Freer R. J., Showell A. H. Some characteristics of the neutrophil receptor for chemotactic peptides. FEBS Lett. 1980 Aug 11;117(1):1–7. doi: 10.1016/0014-5793(80)80900-8. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Smith T. C., Herlihy J. T., Robinson S. C. The effect of the fluorescent probe, 3,3'-dipropylthiadicarbocyanine iodide, on the energy metabolism of Ehrlich ascites tumor cells. J Biol Chem. 1981 Feb 10;256(3):1108–1110. [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. Effects of indomethacin, 5,8,11,14-eicosatetraynoic acid, and p-bromophenacyl bromide on lysosomal enzyme release and superoxide anion generation by human polymorphonuclear leukocytes. Biochem Pharmacol. 1980 Feb 15;29(4):533–538. doi: 10.1016/0006-2952(80)90373-1. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Victor M., Weiss J., Klempner M. S., Elsbach P. Phospholipase A2 activity in the plasma membrane of human polymorphonuclear leukocytes. FEBS Lett. 1981 Dec 28;136(2):298–300. doi: 10.1016/0014-5793(81)80639-4. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Dechatelet L. R., Thomas M. J., O'Flaherty J. T., Waite M. Effect of phagocytosis and ionophores on release and metabolism of arachidonic acid from human neutrophils. Lipids. 1981 Feb;16(2):120–124. doi: 10.1007/BF02535685. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Yorio T., Torres S., Tarapoom N. Alteration in membrane permeability by diacylglycerol and phosphatidylcholine containing arachidonic acid. Lipids. 1983 Jan;18(1):96–99. doi: 10.1007/BF02534698. [DOI] [PubMed] [Google Scholar]


