Abstract
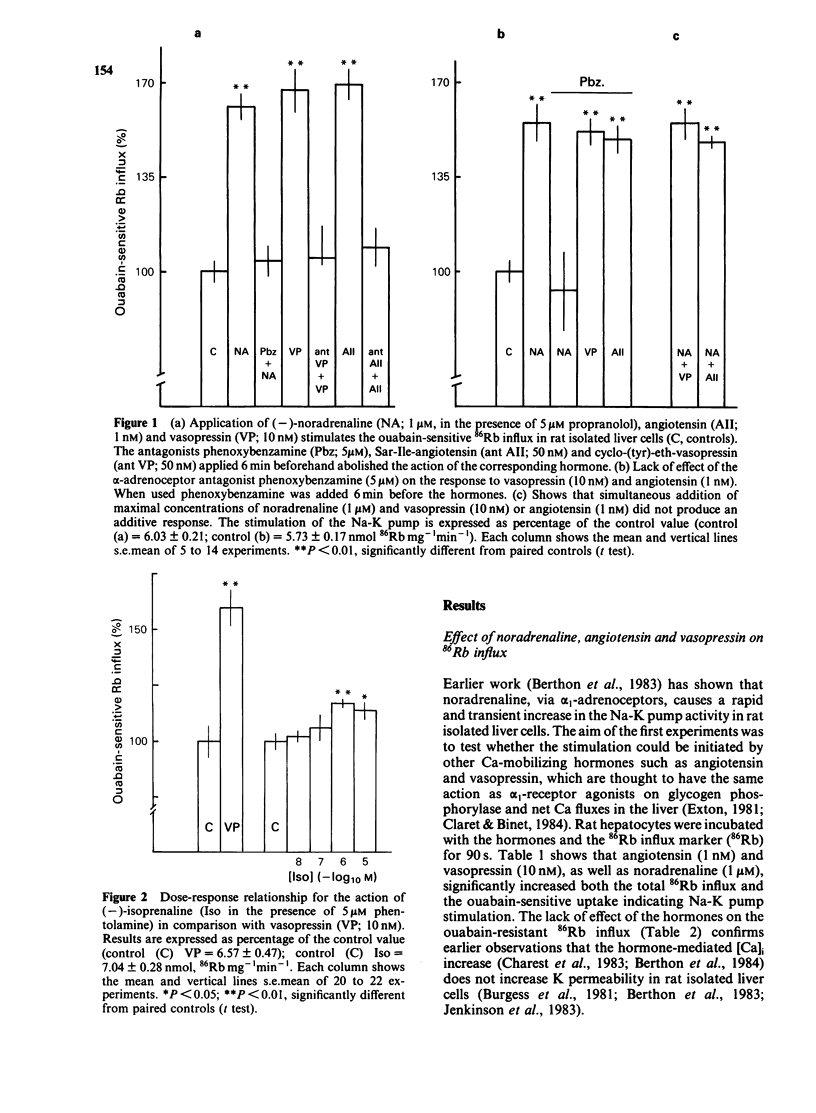
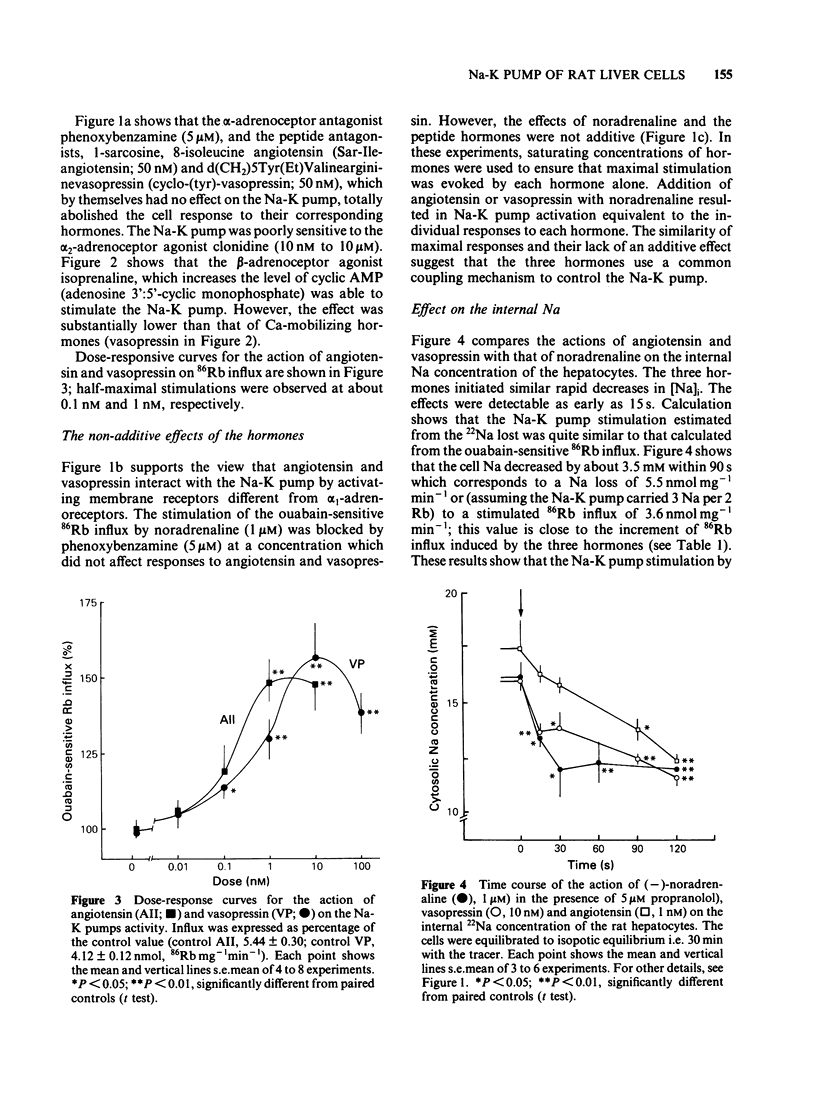
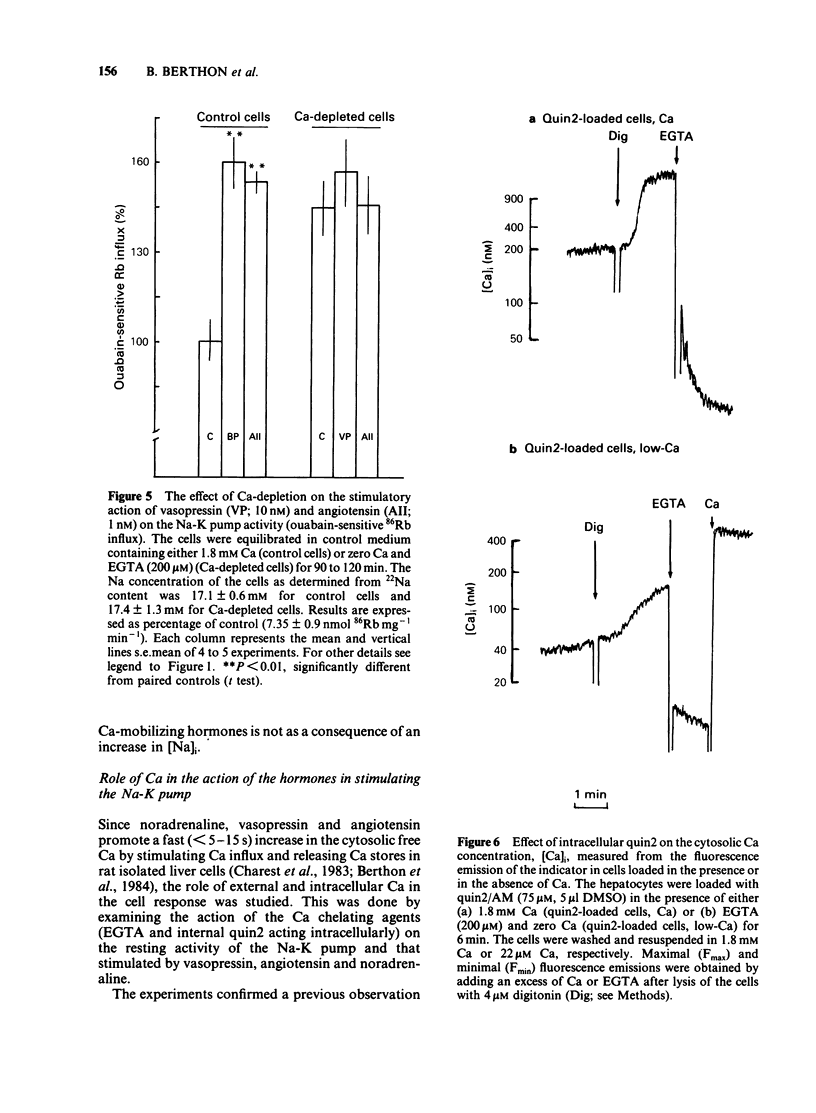
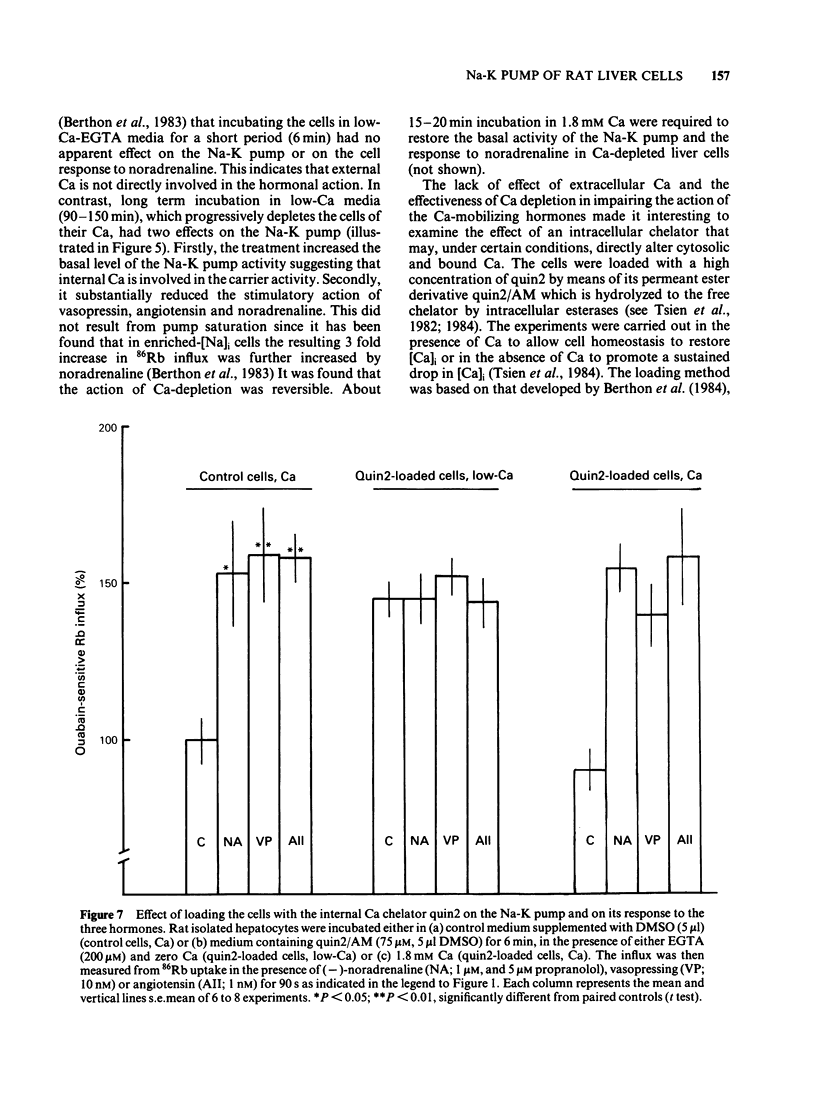
The effects of noradenaline (via alpha 1-adrenoceptors) and of the peptidic hormones vasopressin and angiotensin on the Na-K pump have been studied in rat isolated liver cells. The three hormones increased the cytosolic Ca concentration, stimulated the Na-K pump and decreased the internal Na concentration of the cells. The effects were dose-dependent and were blocked by the corresponding antagonists. The simultaneous addition of maximal doses of noradrenaline and angiotensin or vasopressin were not additive suggesting that the hormones use a common mechanism to stimulate the carrier. Incubating the cells in Ca-free medium for long periods (Ca-depletion) increased the Na-K pump activity and reduced the stimulatory action of vasopressin, angiotensin and noradrenaline. The effect of the Ca indicator quin2, used as an intracellular Ca chelator, was also studied. The cells were loaded with a maximal concentration of [3H]-quin2 acetoxymethyl ester in the presence of external Ca for 6 min. The final cell content was 3.1 nmol quin2 mg-1 cell dry wt. In these cells the cytosolic Ca, as monitored from the fluorescence emission of the indicator, was about 200 nM and Na-K pump activity was normal and the cells remained responsive to the three hormones. Loading the cells with quin2 in the absence of external Ca reduced the [Ca]i from 200 nM to about 40 nM and increased the Na-K pump activity but not as a result of a rise in internal Na concentration. In addition, the rat hepatocytes were no longer sensitive to the hormones. It is proposed that Ca inhibits the Na-K pump by binding the internal sites and that vasopressin, angiotensin and noradrenaline stimulate the carrier by interfering with the inhibitory Ca sites.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J., Jakob A. alpha-Adrenergic stimulation of glycolysis and Na+, K+-transport in perfused rat liver. Eur J Biochem. 1982 Nov 15;128(2-3):293–296. doi: 10.1111/j.1432-1033.1982.tb06964.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Berthon B., Burgess G. M., Capiod T., Claret M., Poggioli J. Mechanism of action of noradrenaline on the sodium-potassium pump in isolated rat liver cells. J Physiol. 1983 Aug;341:25–40. doi: 10.1113/jphysiol.1983.sp014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Claret M., Mazet J. L., Poggioli J. Volume- and temperature-dependent permeabilities in isolated rat liver cells. J Physiol. 1980 Aug;305:267–277. doi: 10.1113/jphysiol.1980.sp013362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanile C. P., Crane J. K., Peach M. J., Garrison J. C. The hepatic angiotensin II receptor. I. Characterization of the membrane-binding site and correlation with physiological response in hepatocytes. J Biol Chem. 1982 May 10;257(9):4951–4958. [PubMed] [Google Scholar]
- Capiod T., Berthon B., Poggioli J., Burgess G. M., Claret M. The effect of Ca2+ -mobilising hormones on the Na+ --K+ pump in isolated rat liver hepatocytes. FEBS Lett. 1982 May 3;141(1):49–52. doi: 10.1016/0014-5793(82)80013-6. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Claret M., Binet A. Mécanismes d'action des hormones mobilisant le calcium dans les hépatocytes de Mammifères. J Physiol (Paris) 1984;79(2):120–128. [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br J Pharmacol. 1980 Apr;68(4):749–755. doi: 10.1111/j.1476-5381.1980.tb10868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaiah D., Ho I. K. Kinetics of catecholamine sensitive Na+-K+ ATPase activity in mouse brain synaptosomes. Biochem Pharmacol. 1977 Nov 1;26(21):2029–2035. doi: 10.1016/0006-2952(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Freychet P. Insulin and glucagon stimulation of (Na+-K+)-ATPase transport activity in isolated rat hepatocytes. J Biol Chem. 1981 Jul 25;256(14):7449–7453. [PubMed] [Google Scholar]
- Friedmann N., Park C. R. Early effects of 3',5'-adenosine monophosphate on the fluxes of calcium end potassium in the perfused liver of normal and adrenalectomized rats. Proc Natl Acad Sci U S A. 1968 Oct;61(2):504–508. doi: 10.1073/pnas.61.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhardt M., Ferry N., Aggerbeck M., Hanoune J. The hepatic alpha 1-adrenergic receptor. Biochem Pharmacol. 1984 Mar 15;33(6):863–868. doi: 10.1016/0006-2952(84)90439-8. [DOI] [PubMed] [Google Scholar]
- Hexum T. D. The effect of catecholamines on transport (Na,K) adenosine triphosphatase. Biochem Pharmacol. 1977 Jul 1;26(13):1221–1227. doi: 10.1016/0006-2952(77)90109-5. [DOI] [PubMed] [Google Scholar]
- Ihlenfeldt M. J. Stimulation of Rb+ transport by glucagon in isolated rat hepatocytes. J Biol Chem. 1981 Mar 10;256(5):2213–2218. [PubMed] [Google Scholar]
- Jakob A., Diem S. Metabolic responses of perfused rat livers to alpha- and beta-adrenergic agonists, glucagon and cyclic AMP. Biochim Biophys Acta. 1975 Sep 8;404(1):57–66. doi: 10.1016/0304-4165(75)90147-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Haylett D. G., Cook N. S. Calcium-activated potassium channels in liver cells. Cell Calcium. 1983 Dec;4(5-6):429–437. doi: 10.1016/0143-4160(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H., Clauser P., Jard S., Morgat J. L. The liver angiotensin receptor involved in the activation of glycogen phosphorylase. Biochem J. 1982 Dec 15;208(3):809–817. doi: 10.1042/bj2080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M. S. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Phillis J. W. Stimulation of cerebral cortical synaptosomal Na-K-ATPase by biogenic amines. Can J Physiol Pharmacol. 1977 Aug;55(4):961–964. doi: 10.1139/y77-130. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Effects of insulin upon ion transport. Biochim Biophys Acta. 1983 Mar 21;737(1):1–49. doi: 10.1016/0304-4157(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Northrop G. Effects of adrenergic blocking agents on epinephrine- and 3',5'-amp-induced responses in the perfused rat liver. J Pharmacol Exp Ther. 1968 Jan;159(1):22–28. [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The contribution of both extracellular and intracellular calcium to the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1984 May 15;220(1):35–42. doi: 10.1042/bj2200035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawas A. H., Gilbert J. C. Effects of adrenergic agonists and antagonists and of the catechol nucleus on the Na+, K+-ATPase and Mg2+-ATPase activities of synaptosomes. Biochem Pharmacol. 1981 Jul 1;30(13):1799–1803. doi: 10.1016/0006-2952(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Storm H., Van Hardeveld C., Kassenaar A. A. The influence of hypothyroidism on the adrenergic stimulation of glycogenolysis in perfused rat liver. Biochim Biophys Acta. 1984 Apr 24;798(3):350–360. doi: 10.1016/0304-4165(84)90109-0. [DOI] [PubMed] [Google Scholar]
- Swann A. C. (Na+, K+)-adenosine triphosphatase regulation by the sympathetic nervous system: effects of noradrenergic stimulation and lesions in vivo. J Pharmacol Exp Ther. 1984 Feb;228(2):304–311. [PubMed] [Google Scholar]
- Trachtenberg M. C., Packey D. J., Sweeney T. In vivo functioning of the Na+, K+-activated ATPase. Curr Top Cell Regul. 1981;19:159–217. doi: 10.1016/b978-0-12-152819-5.50022-1. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Putney J. W., Jr Does calcium mediate the increase in potassium permeability due to phenylephrine or angiotensin II in the liver? J Pharmacol Exp Ther. 1978 Dec;207(3):669–676. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]
- Yingst D. R., Hoffman J. F. Ca-induced K transport in human red blood cell ghosts containing arsenazo III. Transmembrane interactions of Na, K, and Ca and the relationship to the functioning Na-K pump. J Gen Physiol. 1984 Jan;83(1):19–45. doi: 10.1085/jgp.83.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krogt J. A., Belfroid R. D. Characterization and localization of catecholamine-susceptible Na-K ATPase activity of rat striatum: studies using catecholamine receptor (ant)agonists and lesion techniques. Biochem Pharmacol. 1980 Mar 15;29(6):857–868. doi: 10.1016/0006-2952(80)90215-4. [DOI] [PubMed] [Google Scholar]


