Abstract
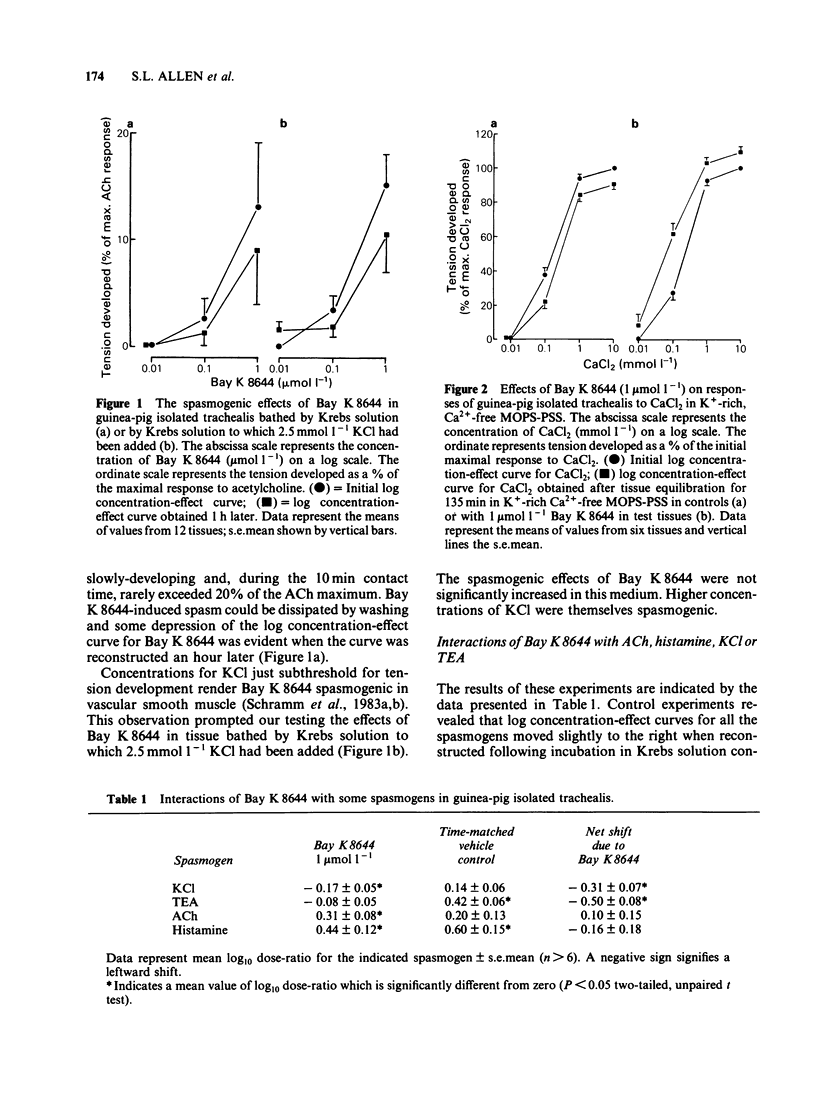
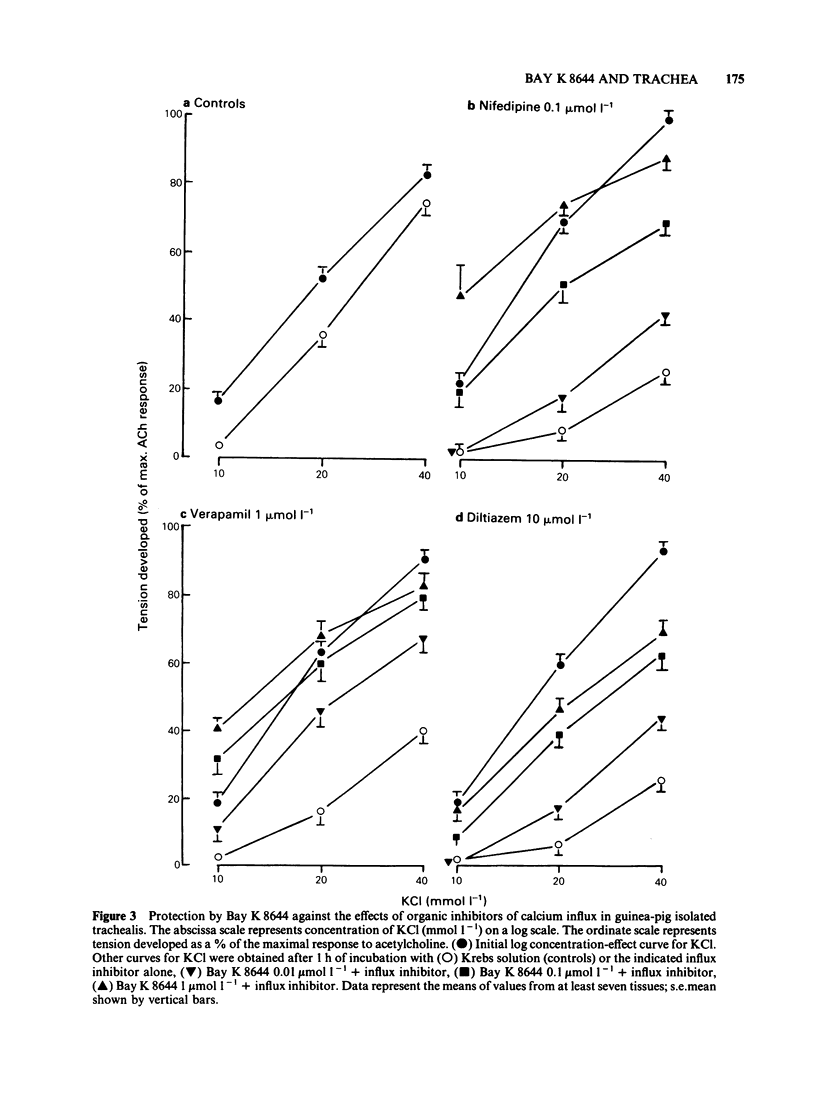
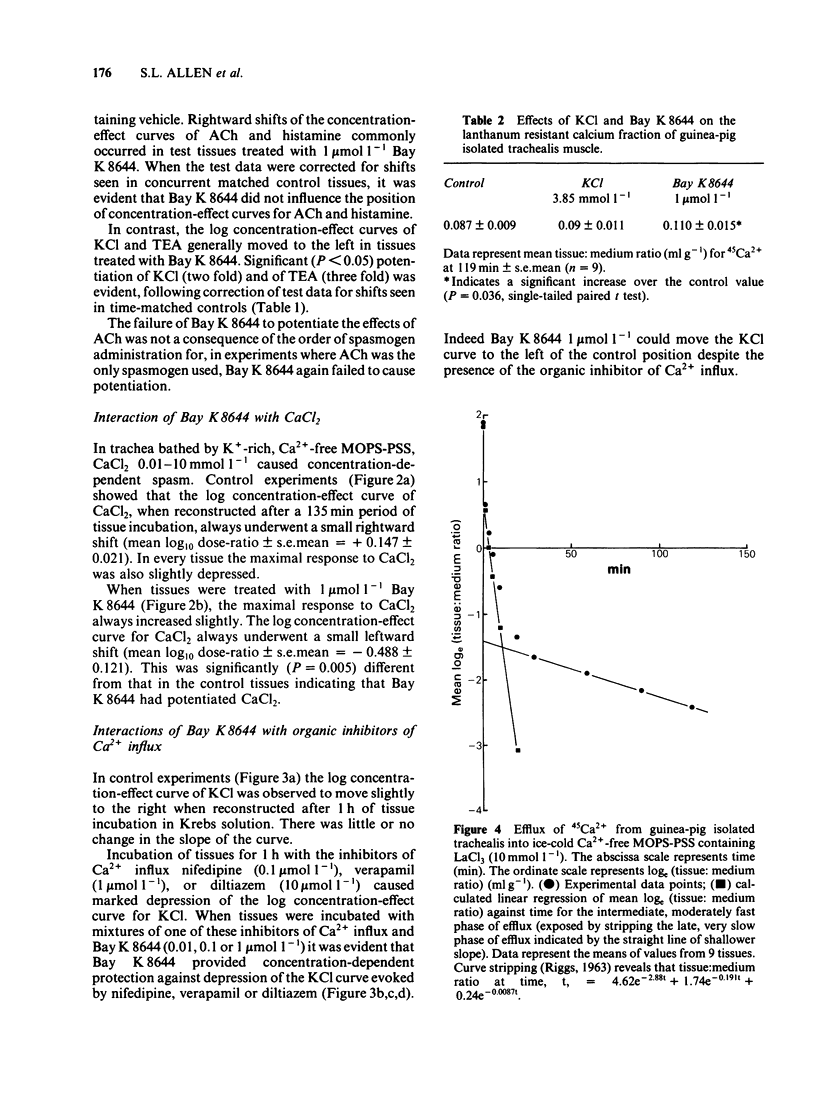
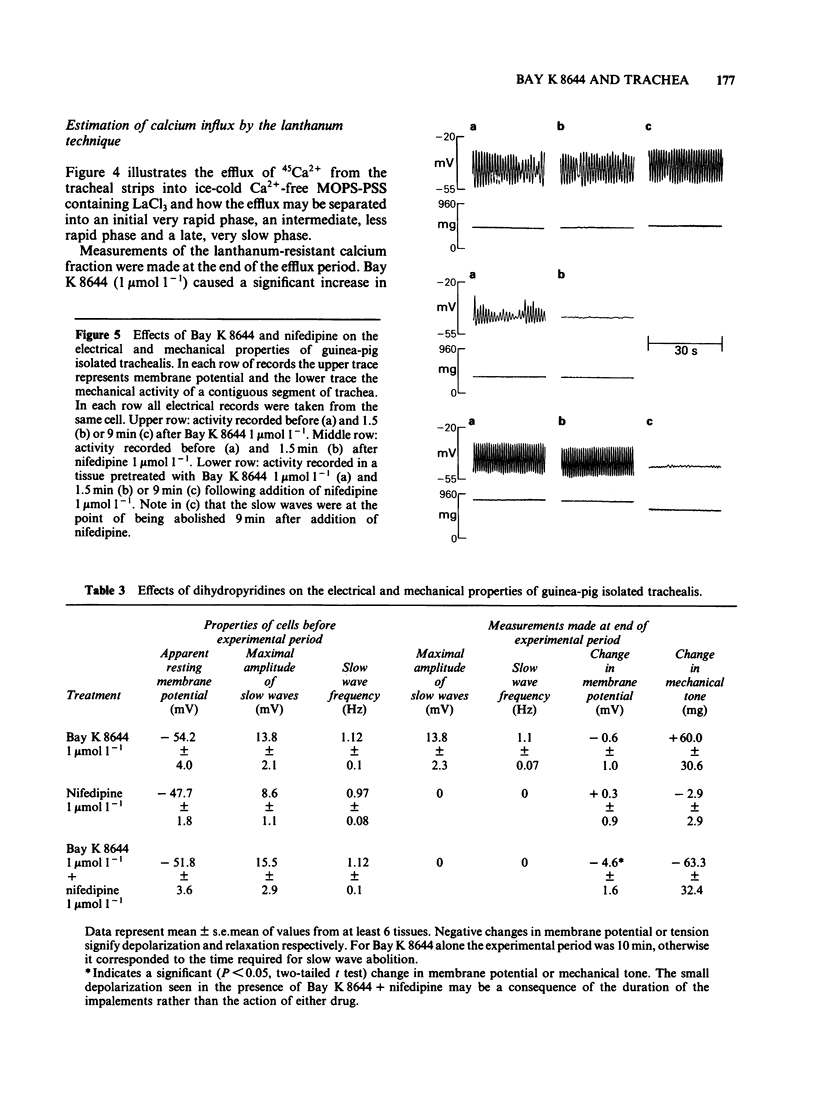
In trachea bathed by Krebs solution containing indomethacin 0.8 mumol l-1, Bay K 8644 (0.01-1 mumol l-1) evoked mild spasm. Peak tension was achieved after 10 min and was generally less than 20% of an acetylcholine (ACh) maximum. The effect of Bay K 8644 was not potentiated by addition of 2.5 mmol l-1 potassium chloride (KCl) to the Krebs solution. Bay K 8644 (1 mumol l-1) caused a small potentiation of KCl and tetraethylammonium (TEA). In contrast it did not modify the actions of ACh or histamine. Bay K 8644 (1 mumol l-1) caused a small potentiation of the effect of calcium chloride (CaCl2) tested in trachea bathed by a K+-rich, Ca2+-free, MOPS-buffered physiological salt solution. Organic inhibitors of calcium influx such as nifedipine (0.1 mumol l-1), verapamil (1 mumol l-1) or diltiazem (10 mumol l-1) each caused marked depression of concentration-effect curves to KCl. Bay K 8644 (0.01-1 mumol l-1) provided concentration-dependent protection against this effect in all three cases. Estimation of calcium influx by the lanthanum technique revealed that Bay K 8644 (1 mumol l-1) was able to promote the cellular influx of Ca2+. Intracellular electrophysiological recording showed that Bay K 8644 (1 mumol l-1) caused no change in the resting membrane potential of trachealis cells and no change in the properties of the spontaneous electrical slow waves. However, Bay K 8644 was able to delay the slow wave suppression evoked by 1 mumol l-1 nifedipine. The ability of Bay K 8644 to promote Ca2+ influx and its ability to protect against the effects of several structurally-unrelated inhibitors of Ca2+ influx are consistent with Bay K 8644 acting as an agonist at the dihydropyridine receptor associated with the voltage-operated Ca2+ channel (VOC) of trachealis muscle. By this action it potentiates those spasmogens (KCl, TEA) which act by permitting Ca2+ influx through VOCs. In contrast it has no effect on those spasmogens (ACh, histamine) which principally act to liberate Ca2+ from intracellular sites of sequestration.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F., Foster R. W., Small R. C. Some effects of nifedipine in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Apr;84(4):861–869. doi: 10.1111/j.1476-5381.1985.tb17380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. S., Small R. C. Evidence of poor conduction of muscle excitation in the longitudinal axis of guinea-pig isolated trachea. Br J Pharmacol. 1983 May;79(1):75–83. doi: 10.1111/j.1476-5381.1983.tb10498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W. A correlation between inhibition of the uptake of 3H from (+)-3H-noradrenaline and potentiation of the responses to (-)-noradrenaline in the guinea-pig isolated trachea. Br J Pharmacol Chemother. 1968 Jun;33(2):357–367. doi: 10.1111/j.1476-5381.1968.tb00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W., Okpalugo B. I., Small R. C. Antagonism of Ca2+ and other actions of verapamil in guinea-pig isolated trachealis. Br J Pharmacol. 1984 Mar;81(3):499–507. doi: 10.1111/j.1476-5381.1984.tb10103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W., Small R. C., Weston A. H. Evidence that the spasmogenic action of tetraethylammonium in guinea-pig trachealis is both direct and dependent on the cellular influx of calcium ion. Br J Pharmacol. 1983 May;79(1):255–263. doi: 10.1111/j.1476-5381.1983.tb10519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J. Calcium channel activation: a different type of drug action. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5580–5583. doi: 10.1073/pnas.81.17.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Levy S., Nasi E., Tillotson D. Intracellular calcium measured with calcium-sensitive micro-electrodes and Arsenazo III in voltage-clamped Aplysia neurones. J Physiol. 1984 Aug;353:127–142. doi: 10.1113/jphysiol.1984.sp015327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Jetley M., Weston A. H. Some effects of sodium nitroprusside, methoxyverapamil (D600) and nifedipine on rat portal vein. Br J Pharmacol. 1980 Feb;68(2):311–319. doi: 10.1111/j.1476-5381.1980.tb10420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Itoh T., Kuriyama H. Agonist actions of Bay K 8644, a dihydropyridine derivative, on the voltage-dependent calcium influx in smooth muscle cells of the rabbit mesenteric artery. J Pharmacol Exp Ther. 1984 Dec;231(3):717–723. [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Activation of calcium channels by novel 1,4-dihydropyridines. A new mechanism for positive inotropics or smooth muscle stimulants. Arzneimittelforschung. 1983;33(9):1268–1272. [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Small R. C. Electrical slow waves and tone of guinea-pig isolated trachealis muscle: effects of drugs and temperature changes. Br J Pharmacol. 1982 Sep;77(1):45–54. doi: 10.1111/j.1476-5381.1982.tb09267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M. Assessment of "Ca2+ -antagonist" effects of drugs in K+ -depolarized smooth muscle. Differentiation of antagonist subgroups. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):234–240. doi: 10.1007/BF00500485. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hwang O., Van Breemen C. Bay K8644 differentiates between potential and receptor operated Ca2+ channels. Eur J Pharmacol. 1984 Jul 20;102(3-4):555–557. doi: 10.1016/0014-2999(84)90581-8. [DOI] [PubMed] [Google Scholar]


