Abstract
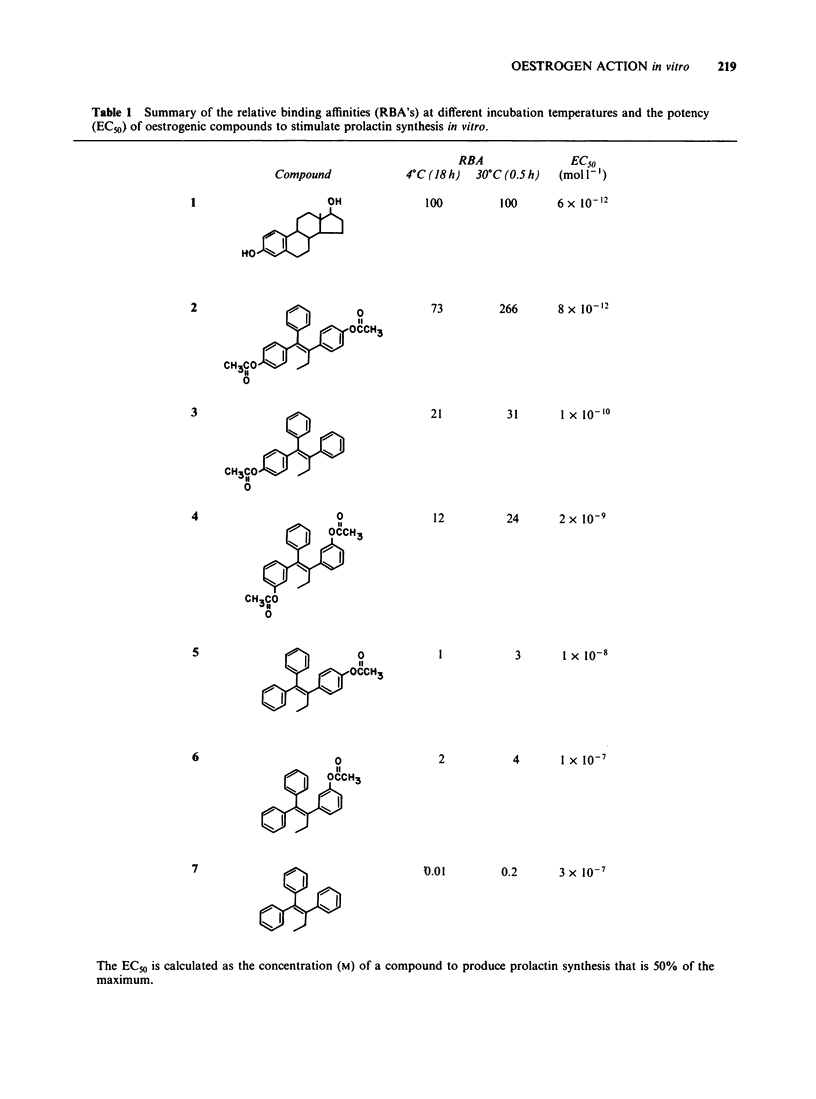
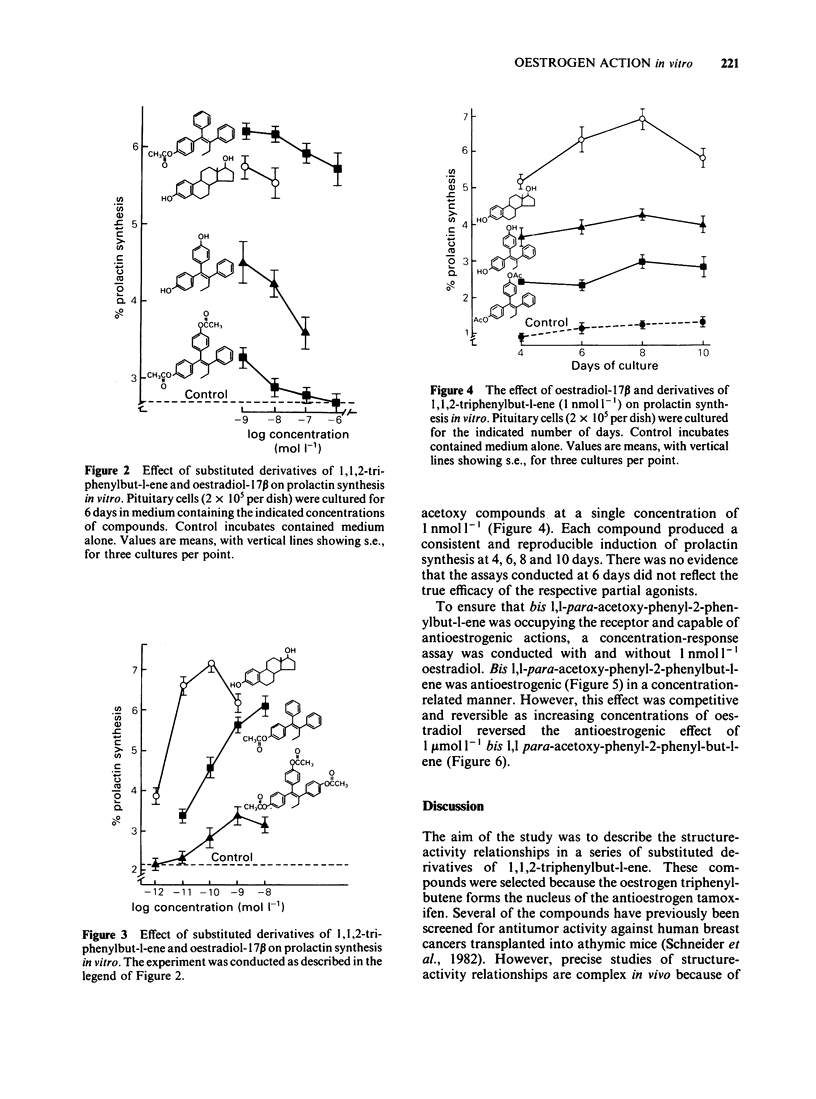
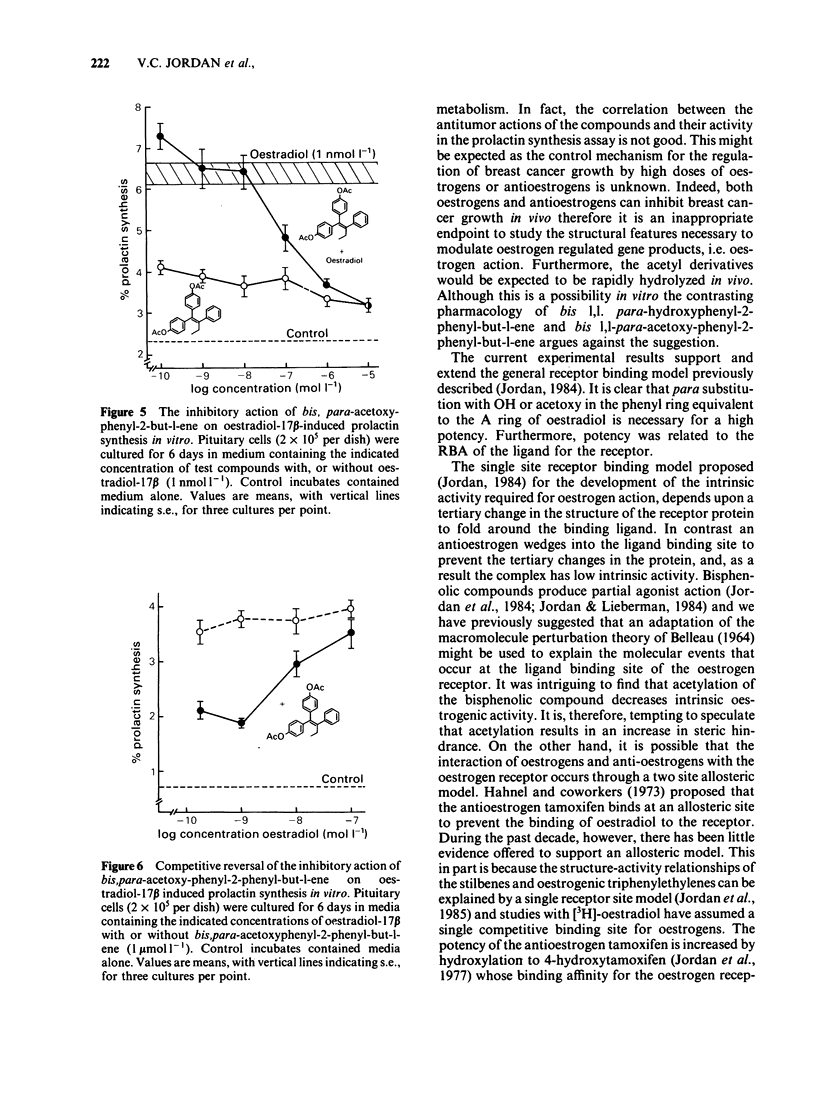
The oestrogenic and antioestrogenic activities of a series of substituted derivatives of 1,1,2 triphenylbut-1-ene have been determined using primary cultures of rat pituitary gland cells to monitor prolactin synthesis in vitro. The relative binding affinity of the agonists for the oestrogen receptor was consistent with their oestrogenic potency. Bis para substitution at C1 of 1,1,2 triphenylbut-1-ene with either phenolic or acetoxy groups produced partial agonists. The antioestrogenic properties were reversible by the incubation of cells with increasing concentrations of oestradiol. The results lend support to a hypothetical single binding site model of oestrogen action, based upon an adaptation of Belleau's macromolecular perturbation theory.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLEAU B. A MOLECULAR THEORY OF DRUG ACTION BASED ON INDUCED CONFORMATIONAL PERTURBATIONS OF RECEPTORS. J Med Chem. 1964 Nov;7:776–784. doi: 10.1021/jm00336a022. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- HOLTKAMP D. E., GRESLIN J. G., ROOT C. A., LERNER L. J. Gonadotrophin inhibiting and anti-fecundity effects of chloramiphene. Proc Soc Exp Biol Med. 1960 Oct;105:197–201. doi: 10.3181/00379727-105-26054. [DOI] [PubMed] [Google Scholar]
- Harper M. J., Walpole A. L. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967 Feb;13(1):101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Costlow M. E., McGuire W. L. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975 Dec;26(6):785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Huppert L. C. Induction of ovulation with clomiphene citrate. Fertil Steril. 1979 Jan;31(1):1–8. doi: 10.1016/s0015-0282(16)43749-0. [DOI] [PubMed] [Google Scholar]
- Hähnel R., Twaddle E., Ratajczak T. The influence of synthetic anti-estrogens on the binding of tritiated estradiol-17beta by cytosols of human uterus and human breast carcinoma. J Steroid Biochem. 1973 Nov;4(6):687–695. doi: 10.1016/0022-4731(73)90044-7. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Lieberman M. E., Cormier E., Koch R., Bagley J. R., Ruenitz P. C. Structural requirements for the pharmacological activity of nonsteroidal antiestrogens in vitro. Mol Pharmacol. 1984 Sep;26(2):272–278. [PubMed] [Google Scholar]
- Jordan V. C., Lieberman M. E. Estrogen-stimulated prolactin synthesis in vitro. Classification of agonist, partial agonist, and antagonist actions based on structure. Mol Pharmacol. 1984 Sep;26(2):279–285. [PubMed] [Google Scholar]
- LERNER L. J., HOLTHAUS F. J., Jr, THOMPSON C. R. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958 Sep;63(3):295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- Lieberman M. E., Gorski J., Jordan V. C. An estrogen receptor model to describe the regulation of prolactin synthesis by antiestrogens in vitro. J Biol Chem. 1983 Apr 25;258(8):4741–4745. [PubMed] [Google Scholar]
- Lieberman M. E., Jordan V. C., Fritsch M., Santos M. A., Gorski J. Direct and reversible inhibition of estradiol-stimulated prolactin synthesis by antiestrogens in vitro. J Biol Chem. 1983 Apr 25;258(8):4734–4740. [PubMed] [Google Scholar]
- Lieberman M. E., Maurer R. A., Claude P., Gorski J. Prolactin synthesis in primary cultures of pituitary cells: regulation by estradiol. Mol Cell Endocrinol. 1982 Mar;25(3):277–294. doi: 10.1016/0303-7207(82)90084-3. [DOI] [PubMed] [Google Scholar]
- Lieberman M. E., Maurer R. A., Gorski J. Estrogen control of prolactin synthesis in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5946–5949. doi: 10.1073/pnas.75.12.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Stone R., Gorski J. Cell-free synthesis of a large translation product of prolactin messenger RNA. J Biol Chem. 1976 May 10;251(9):2801–2807. [PubMed] [Google Scholar]
- Schneider M. R., von Angerer E., Schönenberger H., Michel R. T., Fortmeyer H. P. 1,1,2-triphenylbut-1-enes: relationship between structure, estradiol receptor affinity, and mammary tumor inhibiting properties. J Med Chem. 1982 Sep;25(9):1070–1077. doi: 10.1021/jm00351a013. [DOI] [PubMed] [Google Scholar]
- Tate A. C., Greene G. L., DeSombre E. R., Jensen E. V., Jordan V. C. Differences between estrogen- and antiestrogen-estrogen receptor complexes from human breast tumors identified with an antibody raised against the estrogen receptor. Cancer Res. 1984 Mar;44(3):1012–1018. [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]


