Abstract
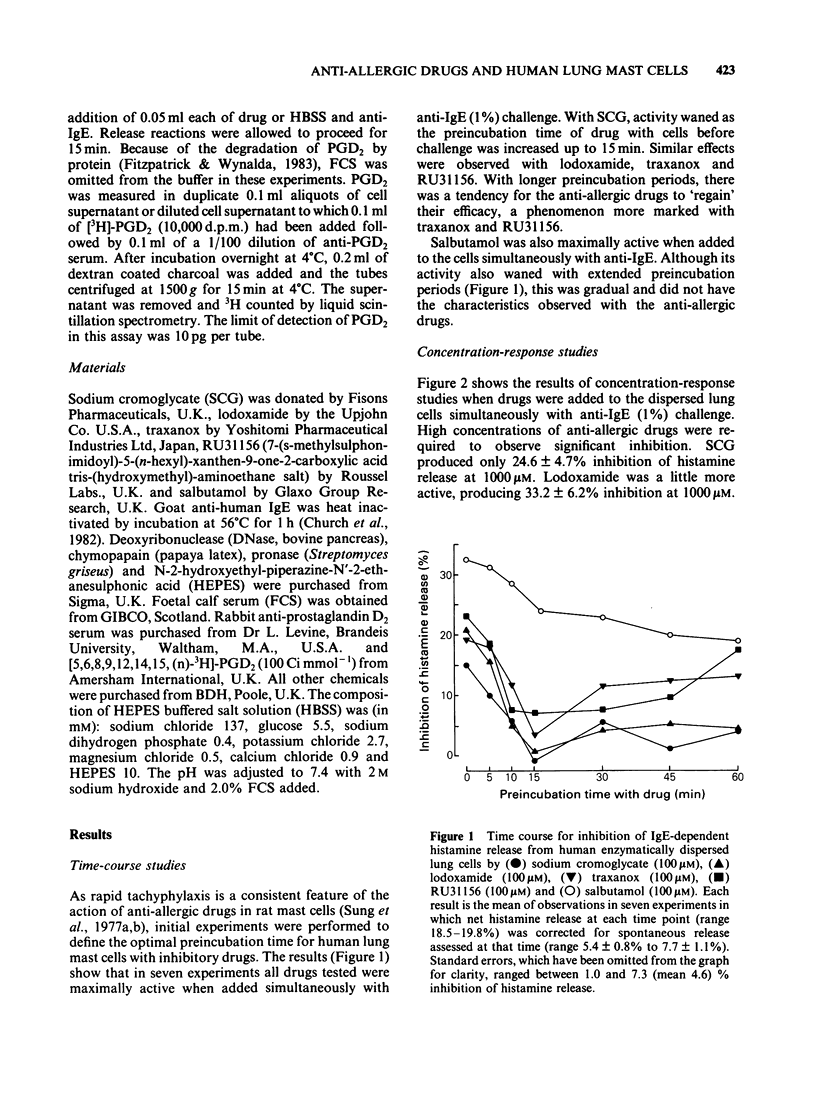
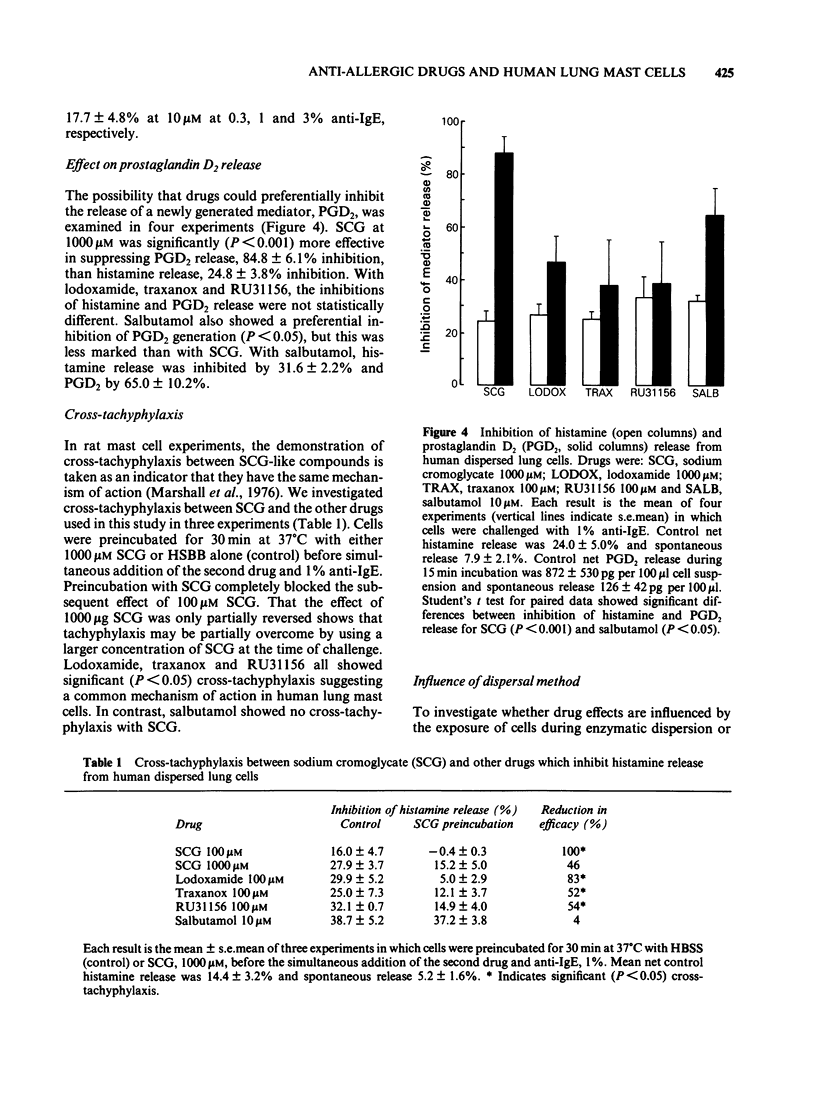
The ability of the anti-allergic drugs, sodium cromoglycate (SCG), lodoxamide, traxanox, RU31156 and the beta-adrenoceptor agonist salbutamol to inhibit IgE-dependent histamine and prostaglandin D2 (PGD2) release was assessed using human dispersed lung mast cells. The anti-allergic drugs were weak inhibitors of histamine release, high concentrations (100-1000 microM) producing less than 35% inhibition. Salbutamol produced 39% inhibition at 10 microM. The efficacy of both SCG and salbutamol was inversely related to the concentration of anti-IgE used for challenge and to the degree of histamine release. Rapid tachyphylaxis was observed with all anti-allergic drugs but not with salbutamol. Cross-tachyphylaxis was observed between SCG and the other anti-allergic drugs, suggesting a common mechanism of action. No cross-tachyphylaxis was observed between SCG and salbutamol. SCG was significantly (P less than 0.001) more effective in inhibiting PGD2 than it was histamine release. Preferential inhibition of PGD2 compared with histamine release was less marked (P less than 0.05) with salbutamol and not significant with the other anti-allergic drugs. Mast cells dispersed by enzymatic digestion of human lung released more histamine on immunological challenge than mechanically dispersed cells obtained by fine chopping of tissue. Enzyme treatment of mechanically dispersed cells removed this difference. Enzymatically and mechanically dispersed cells responded similarly to the inhibitory effects of SCG and salbutamol. Our results suggest that salbutamol is a more effective inhibitor of mediator release from human lung mast cells than anti-allergic drugs. However, with the low levels of mediator release achieved during an allergic reaction in man in vivo, both salbutamol and SCG are likely to be effective inhibitors of both preformed and newly generated mediators.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Richter A. W. Comparison of in vivo and in vitro inhibition of the anaphylactic mechanism by beta-adrenergic stimulants and disodium cromoglycate. Immunology. 1971 Nov;21(5):729–739. [PMC free article] [PubMed] [Google Scholar]
- Atkins P. C., Norman M. E., Zweiman B. Antigen-induced neutrophil chemotactic activity in man. Correlation with bronchospasm and inhibition by disodium cromoglycate. J Allergy Clin Immunol. 1978 Sep;62(3):149–155. doi: 10.1016/0091-6749(78)90099-4. [DOI] [PubMed] [Google Scholar]
- Butchers P. R., Fullarton J. R., Skidmore I. F., Thompson L. E., Vardey C. J., Wheeldon A. A comparison of the anti-anaphylactic activities of salbutamol and disodium cromoglycate in the rat, the rat mast cell and in human lung tissue. Br J Pharmacol. 1979 Sep;67(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Carney I. F. IgE-mediated anaphylactic bronchoconstriction in the guinea pig and the effect of disodium cromoglycate. Int Arch Allergy Appl Immunol. 1976;50(3):322–328. doi: 10.1159/000231509. [DOI] [PubMed] [Google Scholar]
- Church M. K., Pao G. J., Holgate S. T. Characterization of histamine secretion from mechanically dispersed human lung mast cells: effects of anti-IgE, calcium ionophore A23187, compound 48/80, and basic polypeptides. J Immunol. 1982 Nov;129(5):2116–2121. [PubMed] [Google Scholar]
- Church M. K. The role of basophils in asthma. I. Sodium cromoglycate on histamine release and content. Clin Allergy. 1982 May;12(3):223–228. doi: 10.1111/j.1365-2222.1982.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Church M. K., Young K. D. The characteristics of inhibition of histamine release from human lung fragments by sodium cromoglycate, salbutamol and chlorpromazine. Br J Pharmacol. 1983 Apr;78(4):671–679. doi: 10.1111/j.1476-5381.1983.tb09419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg L. S., Church M. K., Holgate S. T. Histamine secretion from human skin slices induced by anti-IgE and artificial secretagogues and the effects of sodium cromoglycate and salbutamol. Clin Allergy. 1985 Jul;15(4):321–328. doi: 10.1111/j.1365-2222.1985.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Cox J. S., Altounyan R. E. Nature and modes of action of disodium cromoglycate (Lomudal). Respiration. 1970;27(Suppl):292–309. doi: 10.1159/000192762. [DOI] [PubMed] [Google Scholar]
- Cox J. S. Disodium cromoglycate (FPL 670) ('Intal'): a specific inhibitor of reaginic antibody-antigen mechanisms. Nature. 1967 Dec 30;216(5122):1328–1329. doi: 10.1038/2161328a0. [DOI] [PubMed] [Google Scholar]
- Damon M., Chavis C., Godard P., Michel F. B., Crastes de Paulet A. Purification and mass spectrometry identification of leukotriene D4 synthesized by human alveolar macrophages. Biochem Biophys Res Commun. 1983 Mar 16;111(2):518–524. doi: 10.1016/0006-291x(83)90337-6. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Wynalda M. A. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J Biol Chem. 1983 Oct 10;258(19):11713–11718. [PubMed] [Google Scholar]
- Flint K. C., Leung K. B., Pearce F. L., Hudspith B. N., Brostoff J., Johnson N. M. Human mast cells recovered by bronchoalveolar lavage: their morphology, histamine release and the effects of sodium cromoglycate. Clin Sci (Lond) 1985 Apr;68(4):427–432. doi: 10.1042/cs0680427. [DOI] [PubMed] [Google Scholar]
- Goose J., Blair A. M. Passive cutaneous anaphylaxis in the rat, induced with two homologous reagin-like antibodies and its specific inhibition with disodium cromoglycate. Immunology. 1969 Jun;16(6):749–760. [PMC free article] [PubMed] [Google Scholar]
- Goto K., Terasawa M., Maruyama Y. Anti-allergic activities of a new benzopyranopyridine derivative Y-12,141 in rats. Int Arch Allergy Appl Immunol. 1979;59(1):13–19. doi: 10.1159/000232234. [DOI] [PubMed] [Google Scholar]
- Hardy C. C., Robinson C., Tattersfield A. E., Holgate S. T. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984 Jul 26;311(4):209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Burns G. B., Robinson C., Church M. K. Anaphylactic- and calcium-dependent generation of prostaglandin D2 (PGD2), thromboxane B2, and other cyclooxygenase products of arachidonic acid by dispersed human lung cells and relationship to histamine release. J Immunol. 1984 Oct;133(4):2138–2144. [PubMed] [Google Scholar]
- Howarth P. H., Durham S. R., Lee T. H., Kay A. B., Church M. K., Holgate S. T. Influence of albuterol, cromolyn sodium and ipratropium bromide on the airway and circulating mediator responses to allergen bronchial provocation in asthma. Am Rev Respir Dis. 1985 Nov;132(5):986–992. doi: 10.1164/arrd.1985.132.5.986. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Moran N. C. Inhibition of the release of histamine from rat mast cells: the effect of cold and adrenergic drugs on release of histamine by compound 48-80 and antigen. J Pharmacol Exp Ther. 1970 Dec;175(3):632–640. [PubMed] [Google Scholar]
- Johnson H. G., VanHout C. A., Wright J. B. Inhibition of allergic reactions by cromoglycate and by a new anti-allergy drug U-42,585E. I. Activity in rats. Int Arch Allergy Appl Immunol. 1978;56(5):416–423. doi: 10.1159/000232051. [DOI] [PubMed] [Google Scholar]
- Kimura I., Moritani Y., Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973 Jun;3(2):195–202. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., DeBernardo R. The immediate allergic response: in vitro action of cyclic AMP-active and other drugs on the two stages of histamine release. J Immunol. 1971 Oct;107(4):1131–1136. [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Schleimer R. P., Peters S. P., Schulman E. S., Adams G. K., 3rd, Newball H. H., Lichtenstein L. M. Generation of leukotrienes by purified human lung mast cells. J Clin Invest. 1982 Oct;70(4):747–751. doi: 10.1172/JCI110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. S., Clement P., Sheridan A. Q., Soryal I., Fairfax A. J., Holgate S. T. Inhaled lodoxamide tromethamine in the treatment of perennial asthma: a double-blind placebo-controlled study. J Allergy Clin Immunol. 1985 Jul;76(1):83–90. doi: 10.1016/0091-6749(85)90808-5. [DOI] [PubMed] [Google Scholar]
- Marquardt D. L., Wasserman S. I. Characterization of the rat mast cell beta-adrenergic receptor in resting and stimulated cells by radioligand binding. J Immunol. 1982 Nov;129(5):2122–2127. [PubMed] [Google Scholar]
- Marshall P. W., Thomson D. S., Evans D. P. The mechansim of tachyphylaxis to ICI 74,917 and disodium cromoglycate. Int Arch Allergy Appl Immunol. 1976;51(3):274–283. doi: 10.1159/000231602. [DOI] [PubMed] [Google Scholar]
- Martin G. L., Atkins P. C., Dunsky E. H., Zweiman B. Effects of theophylline, terbutaline, and prednisone on antigen-induced bronchospasm and mediator release. J Allergy Clin Immunol. 1980 Sep;66(3):204–212. doi: 10.1016/0091-6749(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Miller P., James G. W. Inhibition of experimental immediate hypersensitivity reactions by a novel xanthone, RU 31156. Arch Int Pharmacodyn Ther. 1978 Feb;231(2):328–339. [PubMed] [Google Scholar]
- Pearce F. L., Befus A. D., Gauldie J., Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982 Jun;128(6):2481–2486. [PubMed] [Google Scholar]
- Peters S. P., Schulman E. S., Schleimer R. P., MacGlashan D. W., Jr, Newball H. H., Lichtenstein L. M. Dispersed human lung mast cells. Pharmacologic aspects and comparison with human lung tissue fragments. Am Rev Respir Dis. 1982 Dec;126(6):1034–1039. doi: 10.1164/arrd.1982.126.6.1034. [DOI] [PubMed] [Google Scholar]
- Sung C. P., Saunders H. L., Krell R. D., Chakrin L. W. Studies on the mechanism of tachyphylaxis to disodium cromoglycate. Int Arch Allergy Appl Immunol. 1977;55(1-6):374–384. doi: 10.1159/000231948. [DOI] [PubMed] [Google Scholar]
- Sung C. P., Saunders H. L., Lenhardt E., Chakrin L. W. Further studies on the tachyphylaxis to DSCG. The effects of concentration and temperature. Int Arch Allergy Appl Immunol. 1977;55(1-6):385–394. doi: 10.1159/000231949. [DOI] [PubMed] [Google Scholar]
- Taylor W. A., Roitt I. M. Effect of disodium cromoglycate on various types of anaphylactic reaction in the guinea pig. Int Arch Allergy Appl Immunol. 1973;45(6):795–807. doi: 10.1159/000231080. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C., Sieghart W., Greengard P., Douglas W. W. Antiallergic drug cromolyn may inhibit histamine secretion by regulating phosphorylation of a mast cell protein. Science. 1980 Jan 4;207(4426):80–82. doi: 10.1126/science.6153130. [DOI] [PubMed] [Google Scholar]
- Ting S., Zweiman B., Lavker R. M. Cromolyn does not modulate human allergic skin reactions in vivo. J Allergy Clin Immunol. 1983 Jan;71(1 Pt 1):12–17. doi: 10.1016/0091-6749(83)90540-7. [DOI] [PubMed] [Google Scholar]
- Tung R. S., Lichtenstein L. M. Cyclic AMP agonist inhibition increases at low levels of histamine release from human basophils. J Pharmacol Exp Ther. 1981 Sep;218(3):642–646. [PubMed] [Google Scholar]
- Watt G. D., Bui T. C., Bewtra A. K., Townley R. G. Protective effect opf lodoxamide tromethamine on allergen inhalation challenge. J Allergy Clin Immunol. 1980 Oct;66(4):286–294. doi: 10.1016/0091-6749(80)90023-8. [DOI] [PubMed] [Google Scholar]
- Wells E., Mann J. Phosphorylation of a mast cell protein in response to treatment with anti-allergic compounds. Implications for the mode of action of sodium cromoglycate. Biochem Pharmacol. 1983 Mar 1;32(5):837–842. doi: 10.1016/0006-2952(83)90585-3. [DOI] [PubMed] [Google Scholar]
- Young K. D., Church M. K. Passive anaphylaxis in human lung fragments as a model for testing anti-allergic drugs: its variability and constraints. Int Arch Allergy Appl Immunol. 1983;70(2):138–142. doi: 10.1159/000233311. [DOI] [PubMed] [Google Scholar]


