Abstract
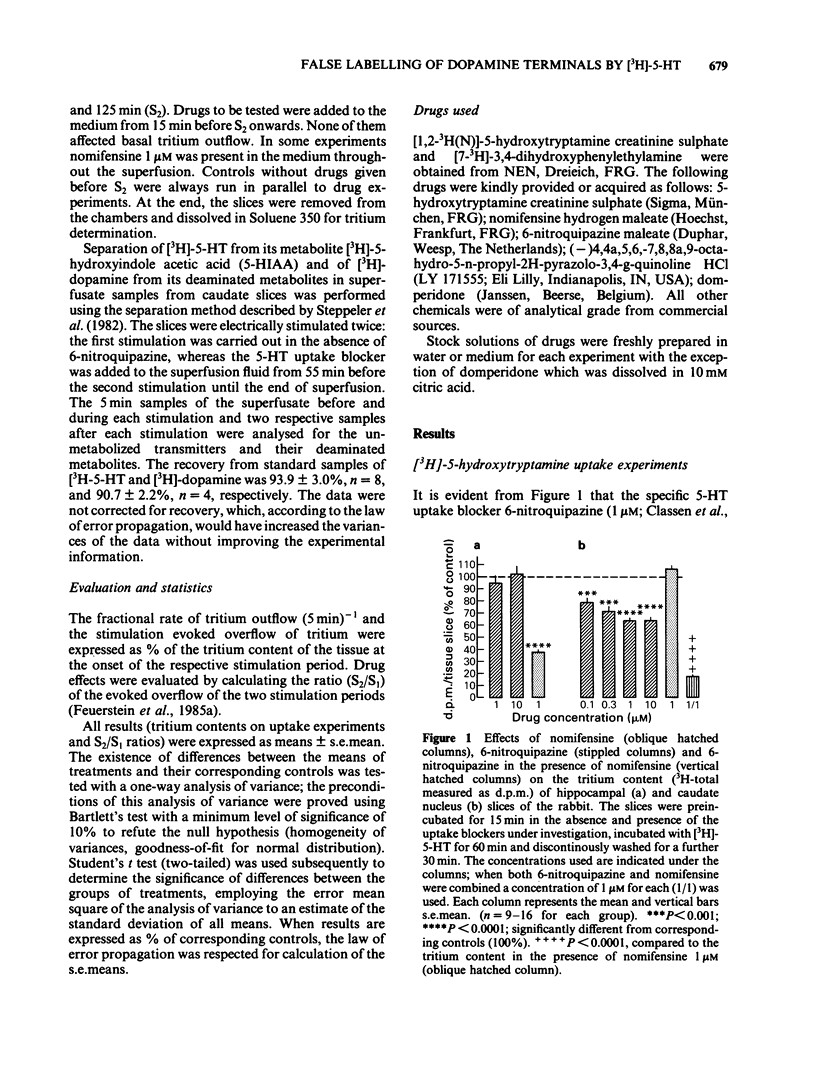
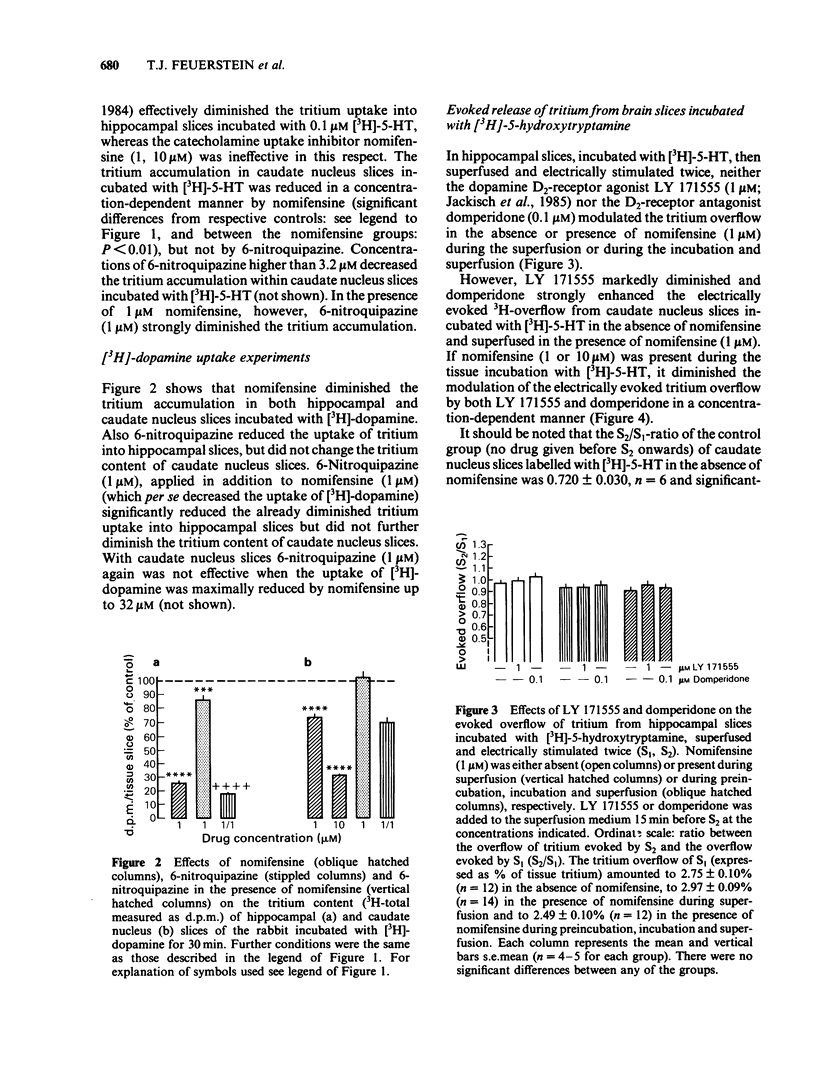
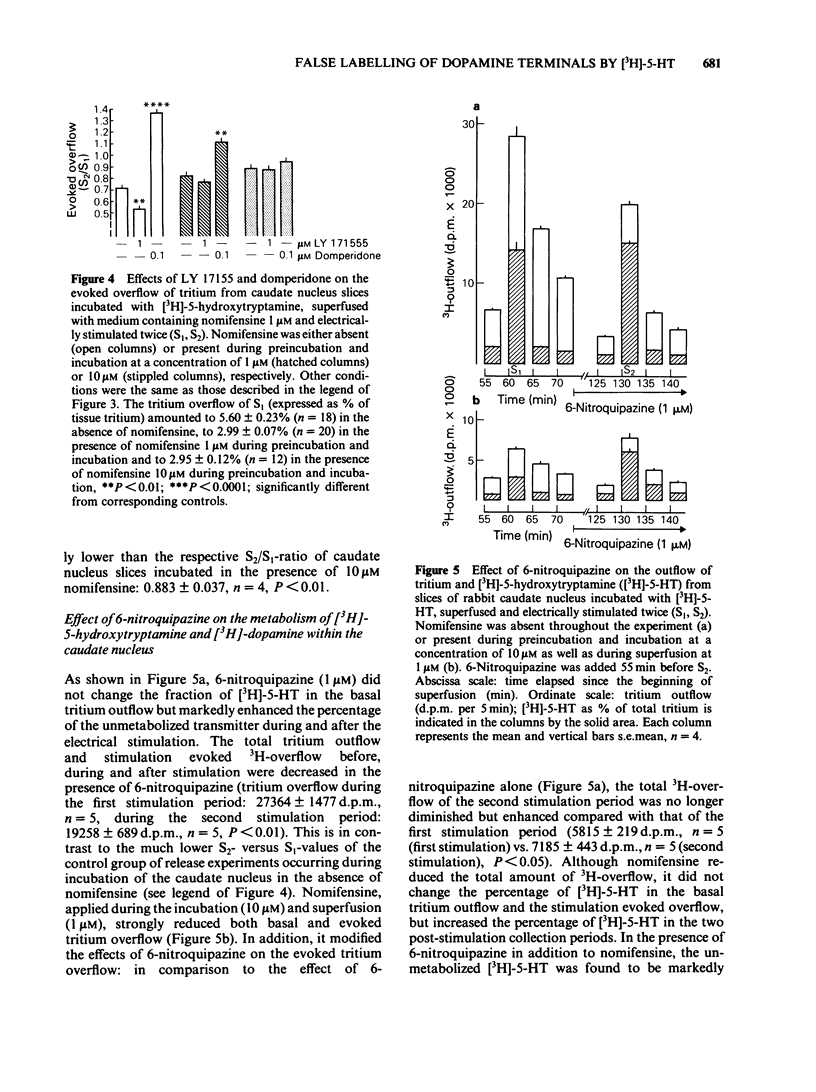
The effect of the catecholamine uptake inhibitor nomifensine and of the 5-hydroxytryptamine (5-HT) uptake blocker 6-nitroquipazine on the accumulation of [3H]-5-HT (0.1 microM, 60 min incubation) and [3H]-dopamine (0.1 microM, 30 min incubation) into slices of hippocampus and caudate nucleus of the rabbit was investigated. In addition, the influence of nomifensine on the electrically evoked [3H]-5-HT release from caudate nucleus slices and of nomifensine and 6-nitroquipazine on [3H]-5-HT released from caudate nucleus slices was analysed. In hippocampal slices, which contain practically no dopaminergic but densely distributed 5-hydroxytryptaminergic and noradrenergic nerve terminals (ratio of dopamine:5-HT:noradrenaline about 1:30:25), nomifensine (1, 10 microM) did not affect the accumulation of [3H]-5-HT; 6-nitroquipazine (1 microM) reduced [3H]-5-HT uptake to about 35% of controls. In the caudate nucleus, however, where dopamine is the predominant monoamine (ratio of dopamine:5-HT:noradrenaline about 400:25:15) nomifensine (1, 10 microM) reduced the tritium accumulation to 65% whereas 6-nitroquipazine (1 microM) was ineffective. The combination of both drugs (1 microM each) led to a further decrease to about 15%. The uptake of [3H]-dopamine into hippocampal slices was blocked by both nomifensine (1 microM) and 6-nitroquipazine (1 microM) whereas in caudate nucleus slices only nomifensine (1, 10 microM) reduced the accumulation of [3H]-dopamine. The combination of both drugs was not more effective than nomifensine alone. The different effects of both uptake inhibitors in the hippocampus and caudate nucleus suggest a neurone specific rather than a substrate specific mode of action.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger B., Glowinski J. Dopamine uptake in serotoninergic terminals in vitro: a valuable tool for the histochemical differentiation of catecholaminergic and serotoninergic terminals in rat cerebral structures. Brain Res. 1978 May 19;147(1):29–45. doi: 10.1016/0006-8993(78)90770-9. [DOI] [PubMed] [Google Scholar]
- Classen K., Göthert M., Schlicker E. Effects of DU 24565 (6-nitroquipazine) on serotoninergic and noradrenergic neurones of the rat brain and comparison with the effects of quipazine. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(3):198–202. doi: 10.1007/BF00505318. [DOI] [PubMed] [Google Scholar]
- Ennis C., Kemp J. D., Cox B. Characterisation of inhibitory 5-hydroxytryptamine receptors that modulate dopamine release in the striatum. J Neurochem. 1981 Apr;36(4):1515–1520. doi: 10.1111/j.1471-4159.1981.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein T. J., Hertting G., Jackisch R. Endogenous noradrenaline as modulator of hippocampal serotonin (5-HT)-release. Dual effects of yohimbine, rauwolscine and corynanthine as alpha-adrenoceptor antagonists and 5-HT-receptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):216–221. doi: 10.1007/BF00501871. [DOI] [PubMed] [Google Scholar]
- Feuerstein T. J., Hertting G., Jackisch R. Modulation of hippocampal serotonin (5-HT) release by endogenous adenosine. Eur J Pharmacol. 1985 Jan 2;107(2):233–242. doi: 10.1016/0014-2999(85)90063-9. [DOI] [PubMed] [Google Scholar]
- Hunt P., Kannengiesser M., Raynaud J. Nomifensine: a new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J Pharm Pharmacol. 1974 May;26(5):370–371. doi: 10.1111/j.2042-7158.1974.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Jackisch R., Moll S., Feuerstein T. J., Hertting G. Dopaminergic modulation of hippocampal noradrenaline release. Evidence for alpha 2-antagonistic effects of some dopamine receptor agonists and antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1985 Aug;330(2):105–113. doi: 10.1007/BF00499902. [DOI] [PubMed] [Google Scholar]
- Juorio A. V., Greenshaw A. J. Tryptamine concentrations in areas of 5-hydroxytryptamine terminal innervation after electrolytic lesions of midbrain raphe nuclei. J Neurochem. 1985 Aug;45(2):422–426. doi: 10.1111/j.1471-4159.1985.tb04004.x. [DOI] [PubMed] [Google Scholar]
- Kelly E., Jenner P., Marsden C. D. Evidence that [3H]dopamine is taken up and released from nondopaminergic nerve terminals in the rat substantia nigra in vitro. J Neurochem. 1985 Jul;45(1):137–144. doi: 10.1111/j.1471-4159.1985.tb05485.x. [DOI] [PubMed] [Google Scholar]
- Lee E. H., Geyer M. A. Similarities of the effects of apomorphine and 3-PPP on serotonin neurons. Eur J Pharmacol. 1983 Oct 28;94(3-4):297–303. doi: 10.1016/0014-2999(83)90418-1. [DOI] [PubMed] [Google Scholar]
- Schacht U., Heptner W. Effect of nomifensine (HOE 984), a new antidepressant, on uptake of noradrenaline and serotonin and on release of noradrenaline in rat brain synaptosomes. Biochem Pharmacol. 1974 Dec 15;23(24):3413–3422. doi: 10.1016/0006-2952(74)90344-x. [DOI] [PubMed] [Google Scholar]
- Schlicker E., Classen K., Göthert M. GABAB receptor-mediated inhibition of serotonin release in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(2):99–105. doi: 10.1007/BF00517304. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Starke K., Reimann W., Zumstein A., Hertting G. Effect of dopamine receptor agonists and antagonists on release of dopamine in the rabbit caudate nucleus in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;305(1):27–36. doi: 10.1007/BF00497003. [DOI] [PubMed] [Google Scholar]
- Starke K., Späth L., Lang J. D., Adelung C. Further functional in vitro comparison of pre- and postsynaptic dopamine receptors in the rabbit caudate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):298–306. doi: 10.1007/BF00512467. [DOI] [PubMed] [Google Scholar]
- Steppeler A., Döring C., Hedler L., Starke K. Effect of amezinium on the release and catabolism of 3H-monoamines in brain slices. Biochem Pharmacol. 1982 Jul 15;31(14):2395–2402. doi: 10.1016/0006-2952(82)90535-4. [DOI] [PubMed] [Google Scholar]
- Strittmatter H., Jackisch R., Hertting G. Role of dopamine receptors in the modulation of acetylcholine release in the rabbit hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(3):195–200. doi: 10.1007/BF00505485. [DOI] [PubMed] [Google Scholar]


