Abstract
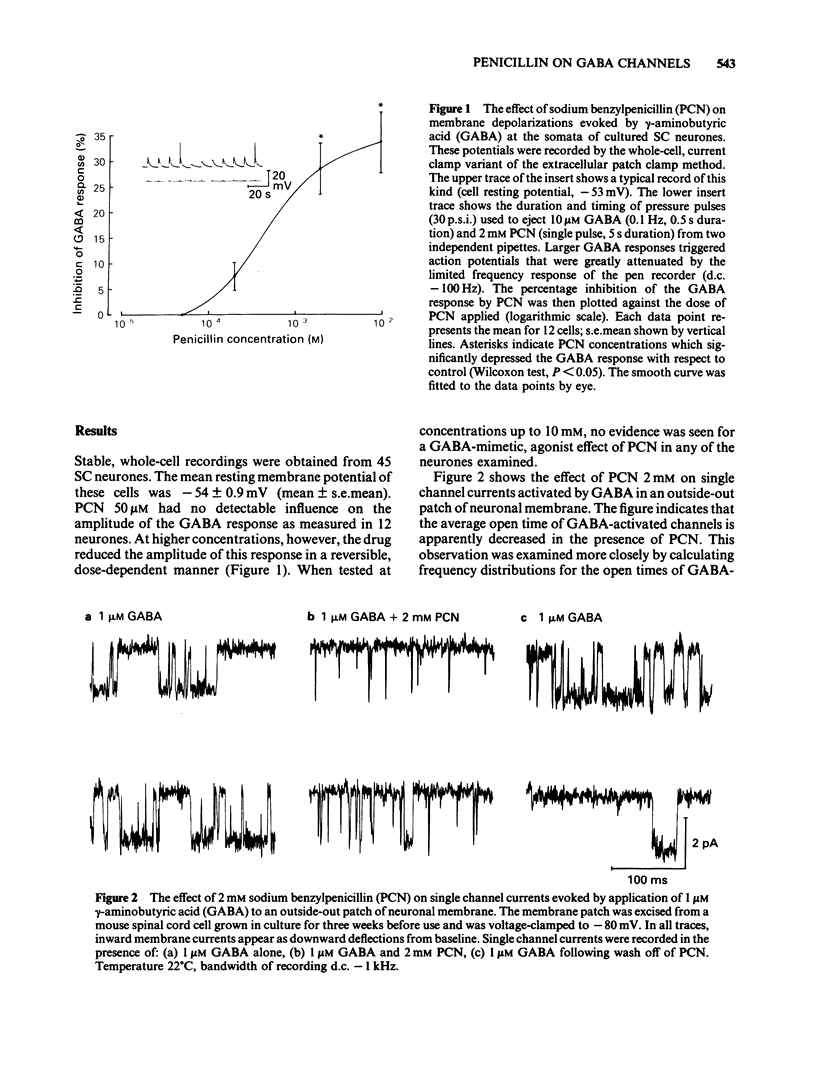
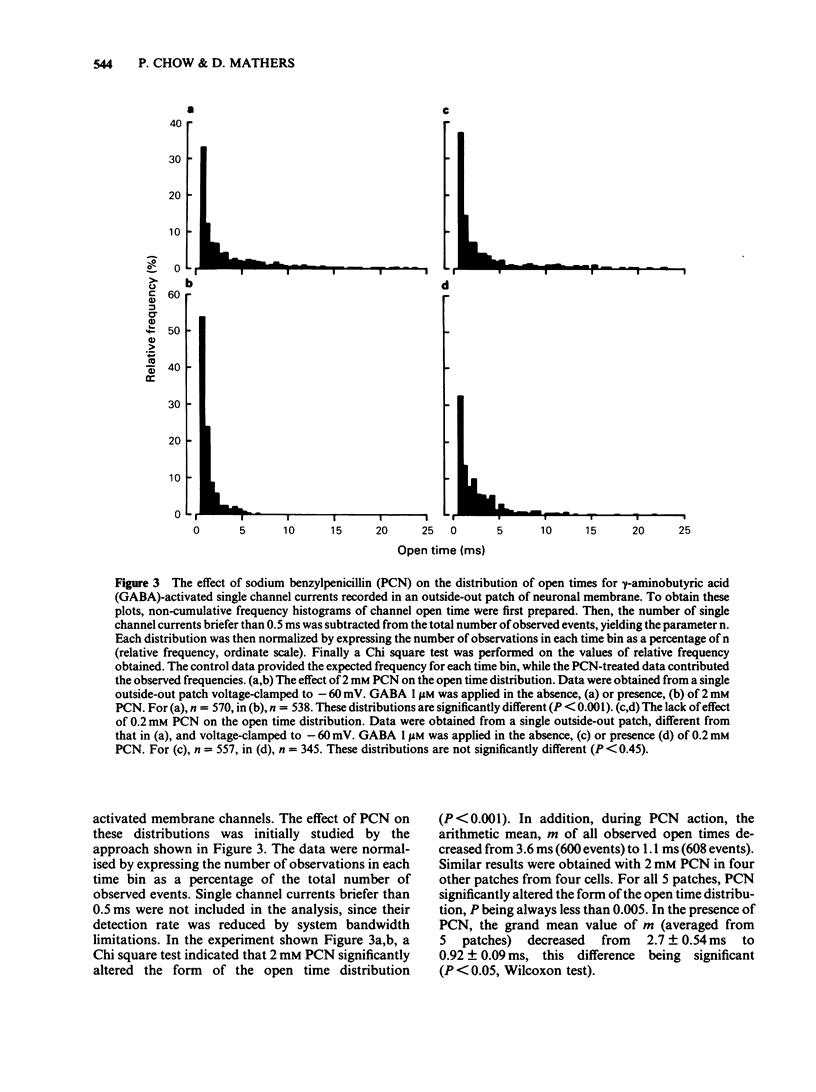
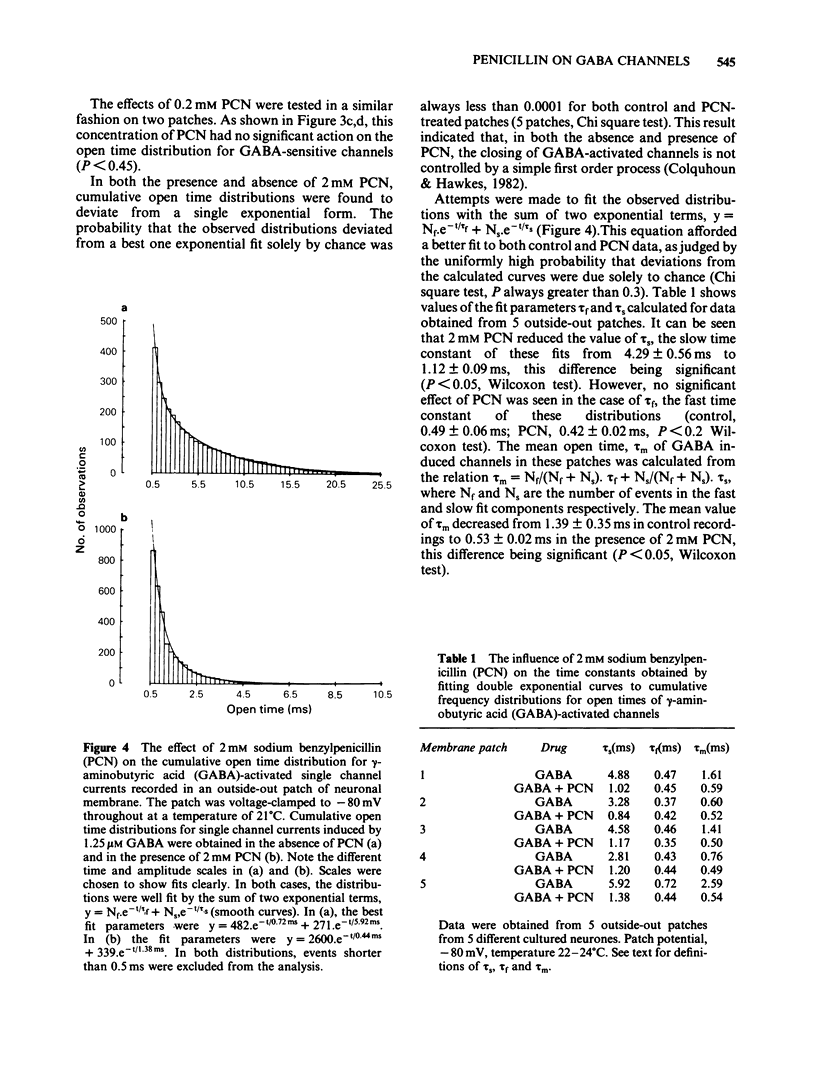
The influence of sodium benzylpenicillin (PCN) on membrane channels activated by gamma-aminobutyric acid (GABA) was studied in cultured spinal neurones of the mouse by the extracellular patch clamp technique. In whole-cell, current clamp recordings, concentrations of PCN above 0.2 mM significantly reduced the amplitude of the GABA response. Single channel currents activated by GABA were studied in outside-out patches of neuronal membrane. In both the absence and presence of PCN, cumulative open time distributions for GABA-activated channels were well fitted by the sum of two exponential terms, characterized by fast (tau f) and slow time constants (tau s). PCN (2mM) reduced the mean value of tau s from 4.29 +/- 0.56 ms (mean +/- s.e. mean) to 1.12 +/- 0.09 ms but had no significant effect on tau f. The mean open time of GABA-activated channels, calculated from the double exponential fits, decreased from 1.39 +/- 0.35 ms to 0.53 +/- 0.02 ms in the presence of 2 mM PCN. The reduced mean open time of GABA-sensitive channels seen in the presence of PCN may contribute to the convulsant action of the drug in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avoli M. Penicillin induced hyperexcitability in the in vitro hippocampal slice can be unrelated to impairment of somatic inhibition. Brain Res. 1984 Dec 3;323(1):154–158. doi: 10.1016/0006-8993(84)90279-8. [DOI] [PubMed] [Google Scholar]
- Barker J. L., MacDonald J. F., Mathers D. A., McBurney R. N., Oertel W. GABA receptor functions in cultured mouse spinal neurons. Adv Biochem Psychopharmacol. 1981;29:281–293. [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Davenport J., Schwindt P. C., Crill W. E. Presynaptic and long-lasting postsynaptic inhibition during penicillin-induced spinal seizures. Neurology. 1978 Jun;28(6):592–597. doi: 10.1212/wnl.28.6.592. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Penicillin and inhibition in the cat spinal cord. Brain Res. 1972 Oct 27;45(2):638–642. doi: 10.1016/0006-8993(72)90498-2. [DOI] [PubMed] [Google Scholar]
- Gjerstad L., Langmoen I. A., Andersen P. Monosynaptic transmission during epileptiform activity induced by penicillin in hippocampal slices in vitro. Acta Physiol Scand. 1981;113(3):355–362. doi: 10.1111/j.1748-1716.1981.tb06907.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature. 1977 Jun 23;267(5613):720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- Macon J. B., King D. W. Penicillin iontophoresis and the responses of somatosensory cortical neurons to amino acids. Electroencephalogr Clin Neurophysiol. 1979 Jul;47(1):52–63. doi: 10.1016/0013-4694(79)90032-4. [DOI] [PubMed] [Google Scholar]
- McBurney R. N., Barker J. L. GABA-induced conductance fluctuations in cultured spinal neurones. Nature. 1978 Aug 10;274(5671):596–597. doi: 10.1038/274596a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles H. G., Simmonds M. A. Antagonism by penicillin of gamma-aminobutyric acid depolarizations at presynaptic sites in rat olfactory cortex and cuneate nucleus in vitro. Neuropharmacology. 1980 Jan;19(1):35–38. doi: 10.1016/0028-3908(80)90163-x. [DOI] [PubMed] [Google Scholar]
- Prince D. A. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]


