Abstract
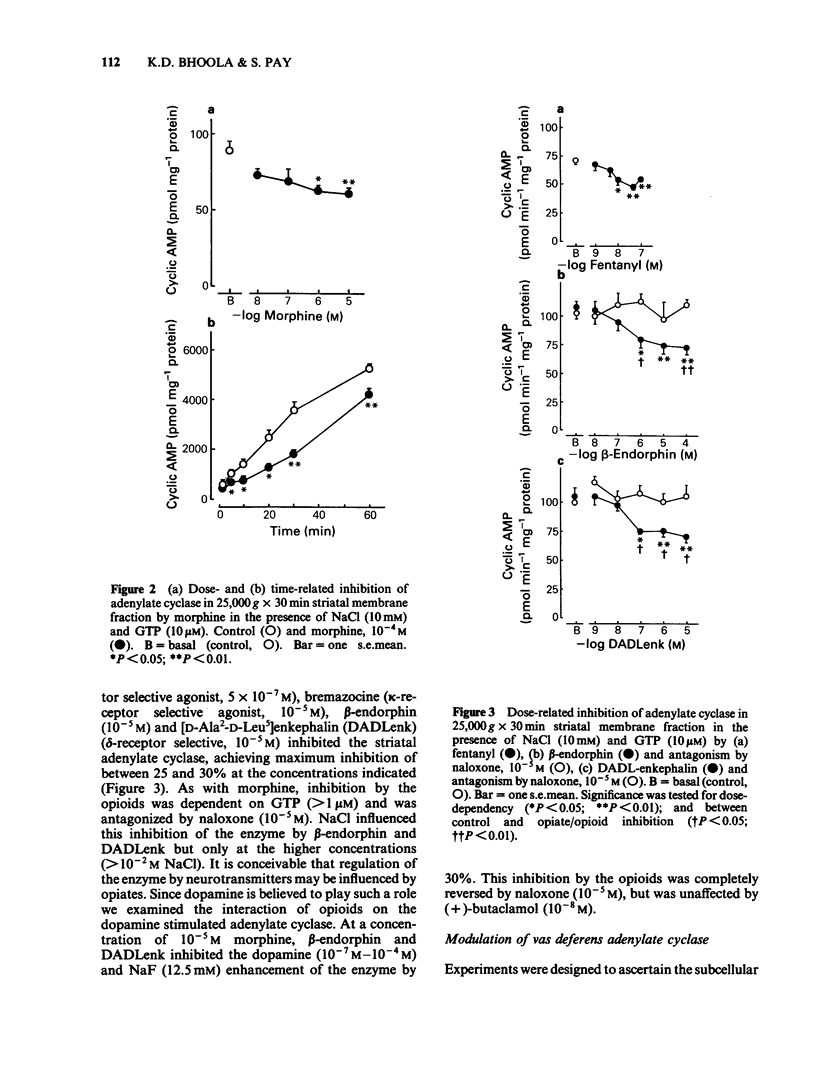
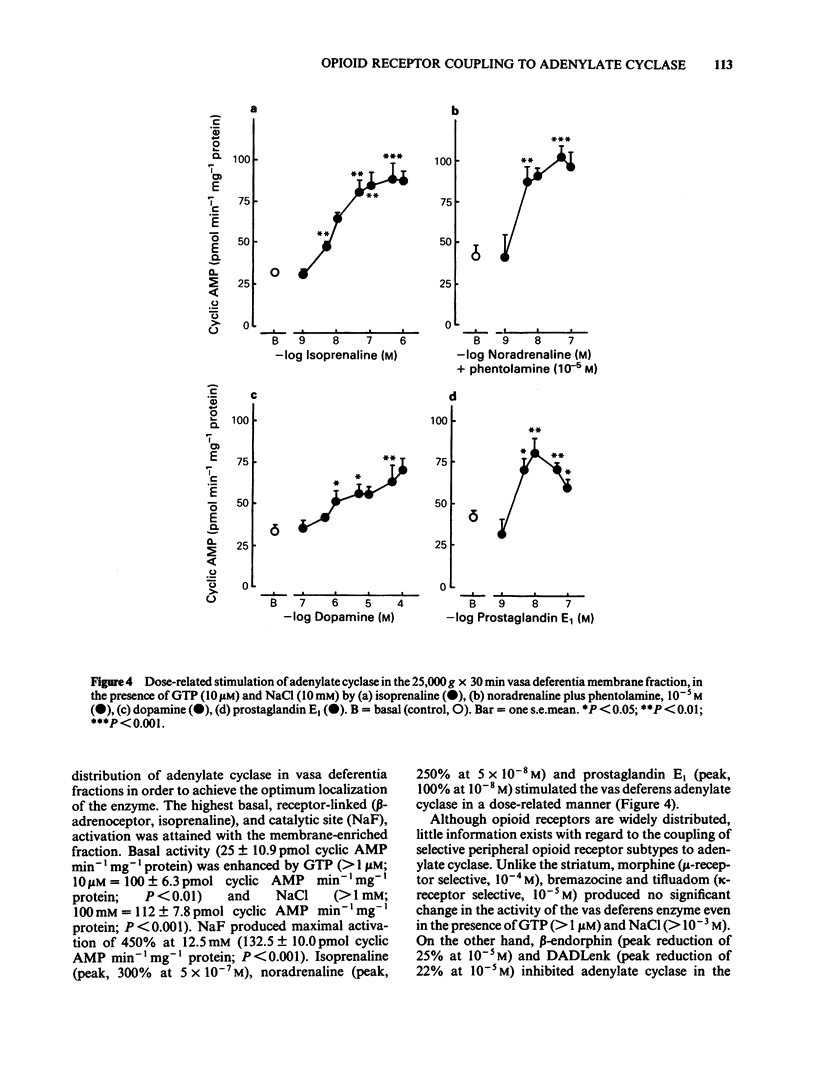
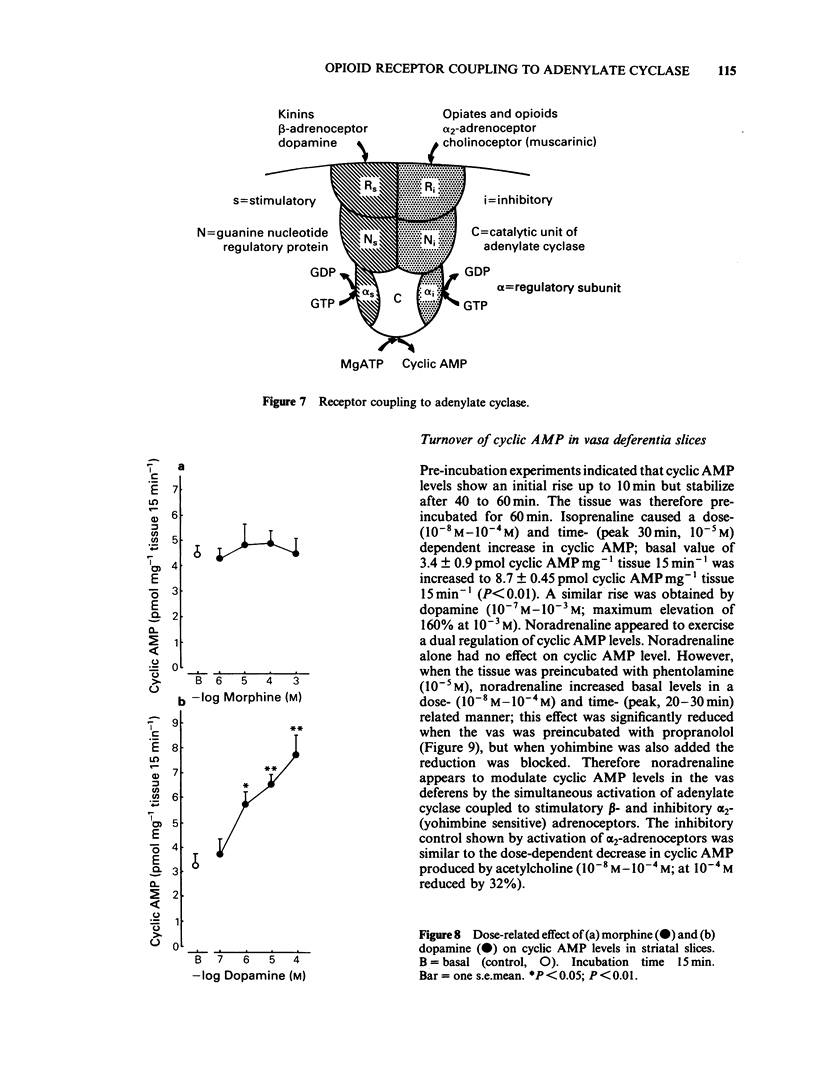
The activity of adenylate cyclase in striatal membrane-enriched fractions (25,000 g) was inhibited by morphine, beta-endorphin, [D-Ala2-D-Leu5] enkephalin (DADLenk), fentanyl and bremazocine. Whereas guanosine triphosphate (GTP) appeared essential for the expression of this effect, sodium chloride seemed to enhance the degree of inhibition. Dopamine stimulation and sodium fluoride activation of the enzyme was also suppressed by morphine, beta-endorphin and DADLenk. beta-Endorphin and DADLenk inhibited adenylate cyclase activity in vasa deferentia membrane-enriched fractions (25,000 g); both opioids required GTP and NaCl and were inhibited by a delta-opioid receptor antagonist and by naloxone. Morphine, bremazocine and tifluadom did not significantly alter the activity of the vas deferens enzyme. Basal cyclic AMP values of striatal slices were not significantly altered by morphine, beta-endorphin or DADLenk. However, dopamine-induced elevation of cyclic AMP was reduced by morphine and this effect of the opiate was suppressed by naloxone. Only beta-endorphin lowered the basal cyclic AMP values in the vas deferens. The physiological relevance of adenylate cyclase coupling to opioid receptor subtypes is considered.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J., Bhoola K. D., Harvey R. F. Intracellular messenger role of cyclic GMP in exocrine pancreas. Nature. 1976 Jul 29;262(5567):404–406. doi: 10.1038/262404a0. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier H. O., Francis D. L. Morphine abstinence is associated with increased brain cyclic AMP. Nature. 1975 May 8;255(5504):159–162. doi: 10.1038/255159b0. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Hypothesis: Inhibition of E prostaglandin-sensitive adenyl cyclase as the mechanism of morphine analgesia. Prostaglandins. 1974 Sep 10;7(5):361–376. doi: 10.1016/0090-6980(74)90100-2. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974 Mar 1;248(5443):24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Londos C., Gill D. L., Rodbell M. Opiate receptor-mediated inhibition of adenylate cyclase in rat striatal plasma membranes. J Neurochem. 1982 Apr;38(4):1164–1167. doi: 10.1111/j.1471-4159.1982.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Govoni S., Kumakura K., Spano P. F., Tonon G. C., Trabucchi M. Interaction of narcotic analgesics with dopamine receptors in rat brain. Pharmacol Res Commun. 1975 Feb;07(1):95–100. doi: 10.1016/s0031-6989(75)80033-6. [DOI] [PubMed] [Google Scholar]
- Guillemin R. Endorphins, brain peptides that act like opiates. N Engl J Med. 1977 Jan 27;296(4):226–228. doi: 10.1056/NEJM197701272960414. [DOI] [PubMed] [Google Scholar]
- Ho I. K., Loh H. H., Way E. L. Cyclic adenosine monophosphate antagonism of morphine analgesia. J Pharmacol Exp Ther. 1973 May;185(2):336–346. [PubMed] [Google Scholar]
- Hughes J., Kosterlitz H. W., Leslie F. M. Proceedings: Assessment of the agonist and antagonist activities of narcotic analgesic drugs by means of the mouse vas deferens. Br J Pharmacol. 1974 May;51(1):139P–140P. [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Sharma S. K., Nirenberg M. Opiate receptors as regulators of adenylate cyclase. Life Sci. 1975 Jun 15;16(12):1869–1874. doi: 10.1016/0024-3205(75)90293-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law P. Y., Wu J., Koehler J. E., Loh H. H. Demonstration and characterization of opiate inhibition of the striatal adenylate cyclase. J Neurochem. 1981 May;36(5):1834–1846. doi: 10.1111/j.1471-4159.1981.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Lemaire S., Magnan J., Regoli D. Rat vas deferens: a specific bioassay for endogenous opioid peptides. Br J Pharmacol. 1978 Nov;64(3):327–329. doi: 10.1111/j.1476-5381.1978.tb08653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P., Iversen L. L. Morphine selectively blocks dopamine-stimulated cyclic AMP formation in rat neostriatal slices [proceedings]. Br J Pharmacol. 1977 Mar;59(3):480P–481P. [PMC free article] [PubMed] [Google Scholar]
- Quik M., Emson P. C., Fahrenkrug J., Iversen L. L. Effect of kainic acid injections and other brain lesions on vasoactive intestinal peptide (VIP)-stimulated formation of cAMP in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):281–286. doi: 10.1007/BF00507115. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening D., Mishra R. K., Makman M. H. Effects of morphine on dopamine-stimulated adenylate cyclase and on cyclic GMP formation in primate brain amygdaloid nucleus. Life Sci. 1976 Oct 15;19(8):1129–1137. doi: 10.1016/0024-3205(76)90247-2. [DOI] [PubMed] [Google Scholar]


