Abstract
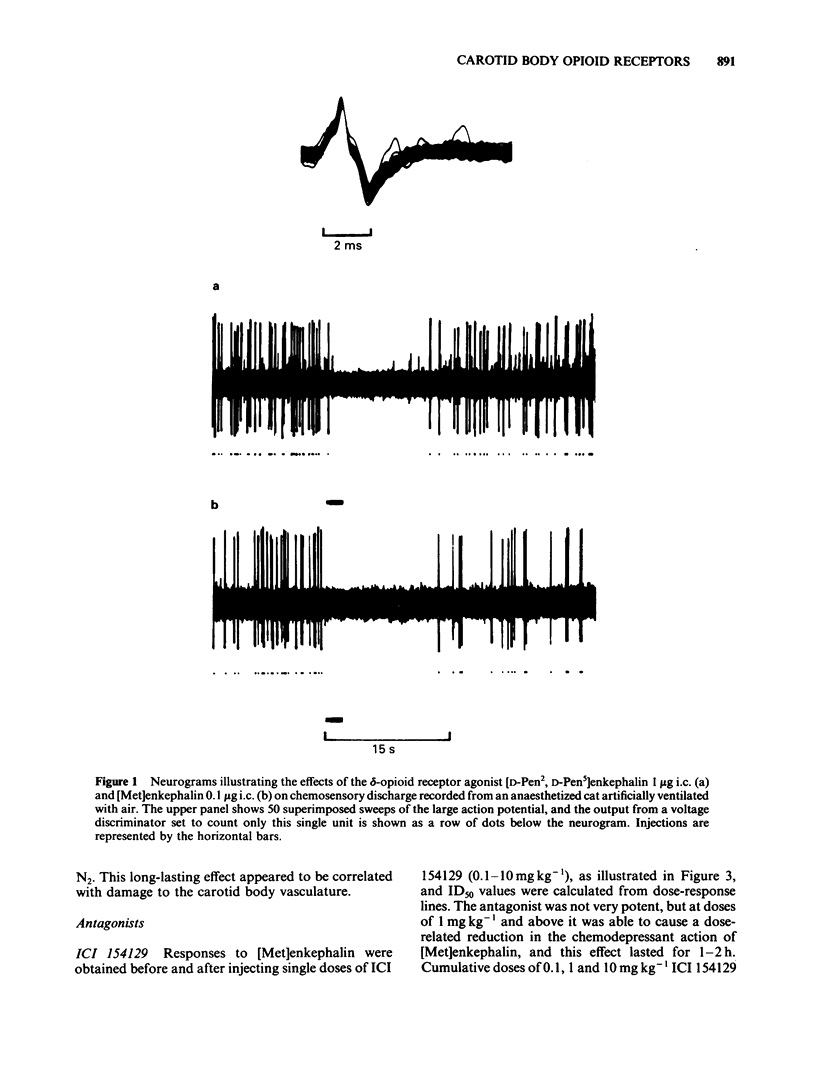
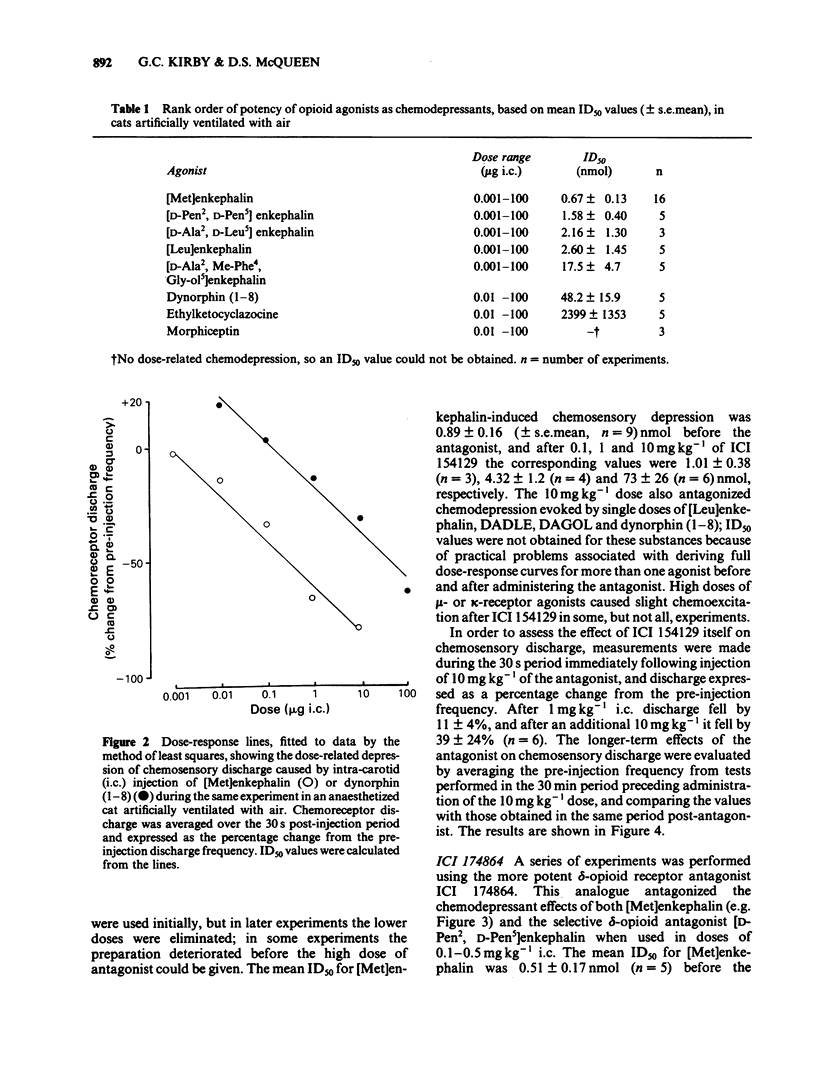
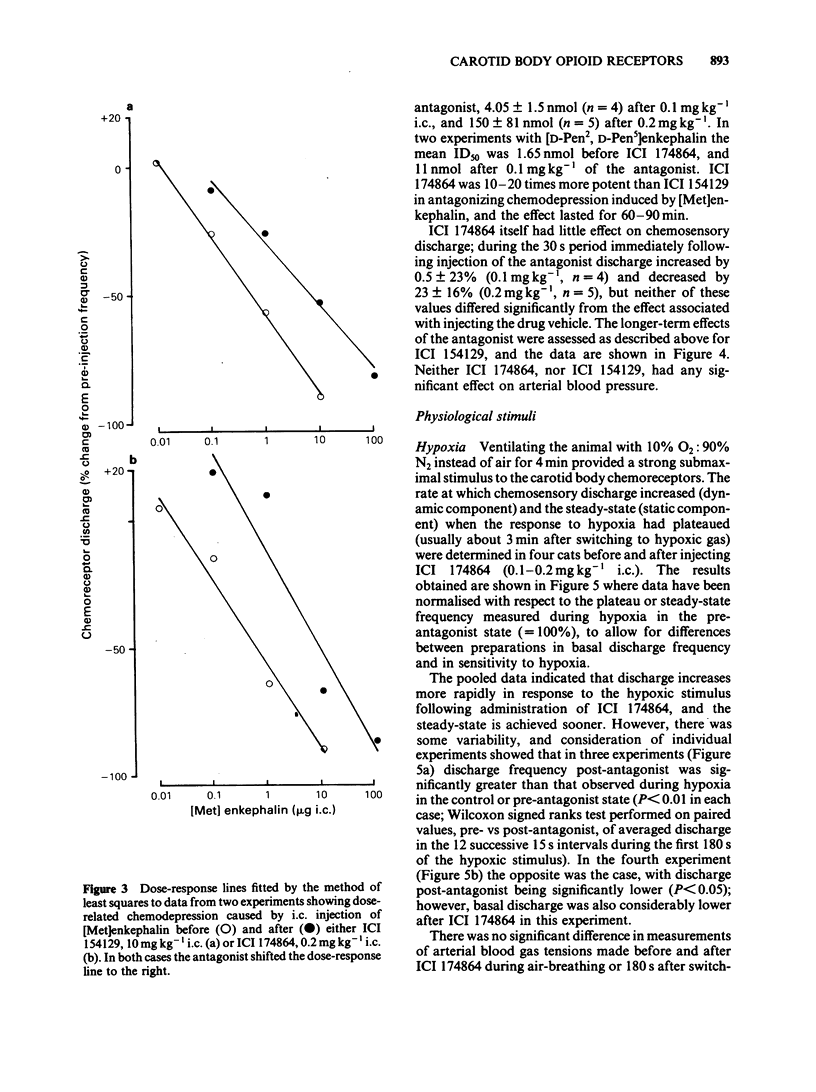
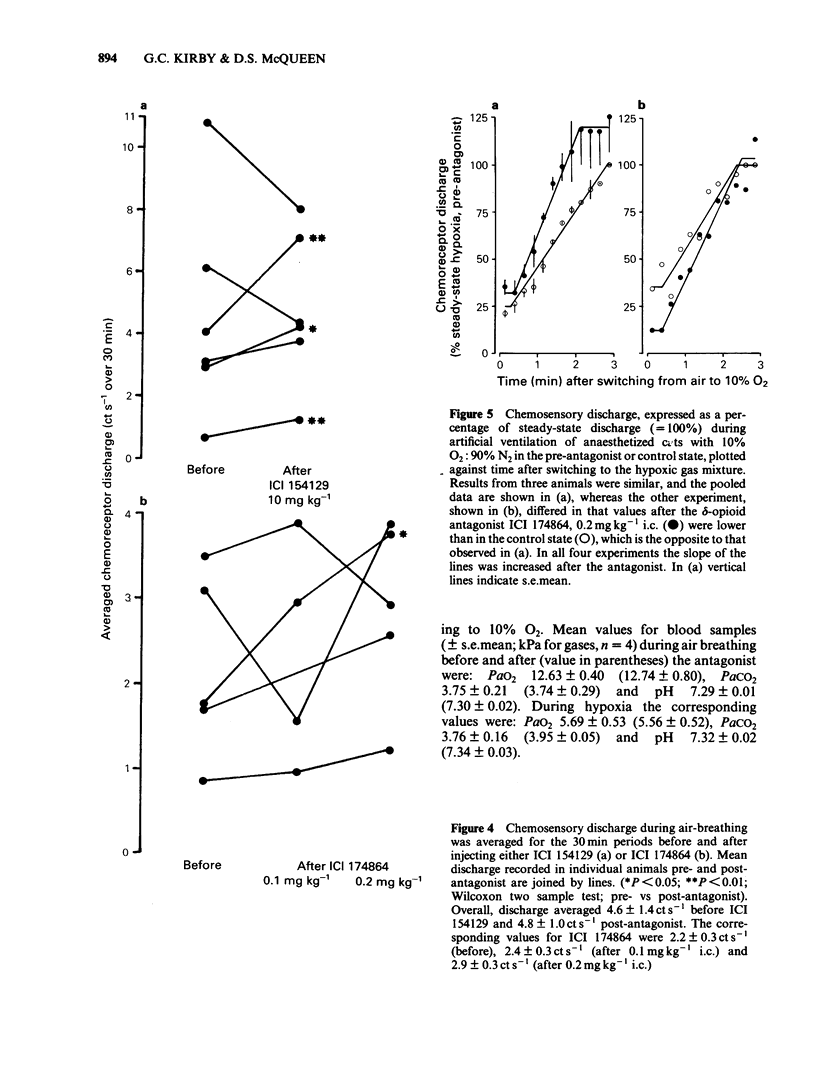
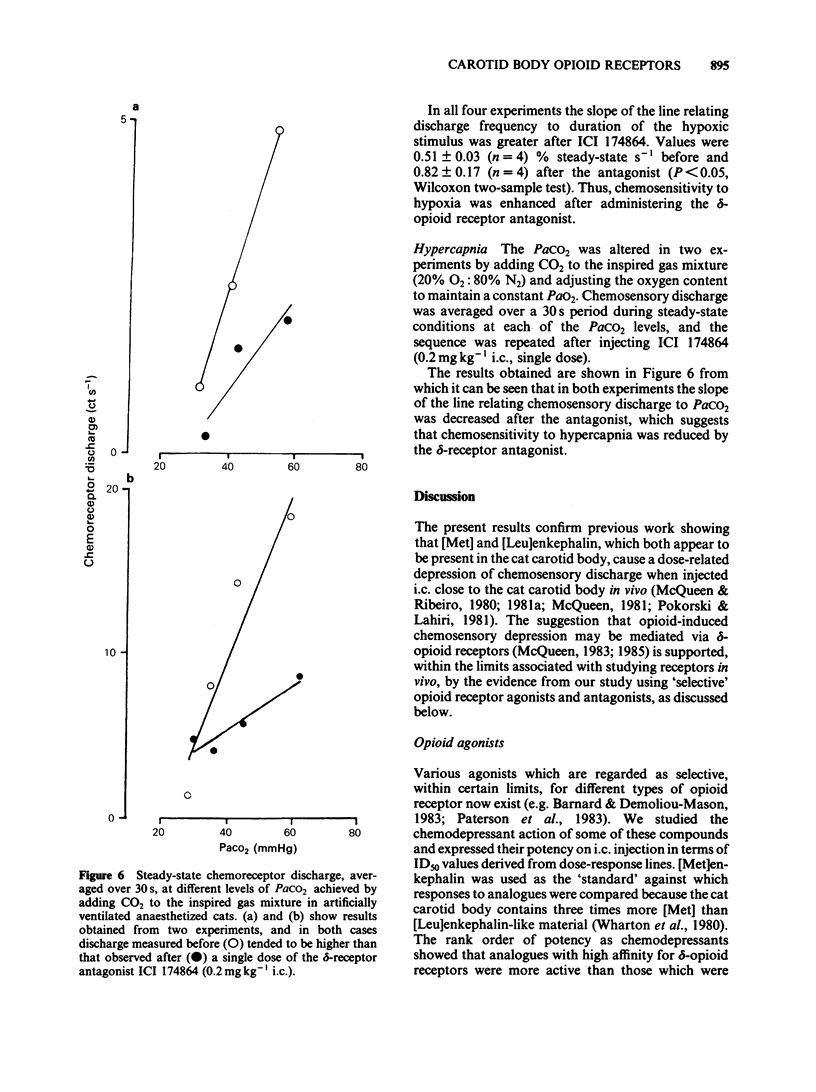
The effects of selective opioid receptor agonists and antagonists on neural discharge recorded from carotid body arterial chemoreceptors in vivo were studied in anaesthetized cats. Mean ID50 values were determined for each agonist and used to assess chemodepressant potency on intracarotid (i.c.) injection in animals artificially ventilated with air. [Met]enkephalin, [Leu]enkephalin, [D-Ala2, D-Leu5]enkephalin and [D-Pen2, D-Pen5]enkephalin were more potent chemodepressants than [D-Ala2, Me-Phe4, Gly-ol5]enkephalin, dynorphin (1-8) or ethylketocyclazocine; morphiceptin (mu-agonist) was inactive. The rank order of potency was compatible with the involvement of delta-opioid receptors in opioid-induced depression of chemosensory discharge. ICI 154129, a delta-opioid receptor antagonist, was used in fairly high doses and caused reversible dose-related antagonism of chemodepression induced by [Met]enkephalin. It also antagonized depression caused by single doses of [Leu]enkephalin, [D-Ala2, D-Leu5]enkephalin, [D-Ala2, Me-Phe4, Gly-ol5]enkephalin or dynorphin (1-8). ICI 174864, a more potent and selective delta-opioid receptor antagonist, also antagonized chemodepression induced by [Met]enkephalin or by the selective delta-receptor agonist [D-Pen2, D-Pen5]enkephalin. Comparison of background or 'spontaneous' chemosensory discharge during the 30 min periods immediately before and after injecting ICI 174864 (0.1-0.2 mg kg-1 i.c.) showed a significant increase in discharge in one experiment, but in four others discharge was either unaffected or decreased after the antagonist, which argues against a toxic depression of chemosensors by endogenous opioids under resting conditions in our preparation. Sensitivity of the carotid chemoreceptors to hypoxia (ventilating with 10% O2) was increased significantly after ICI 174864, which could be taken as evidence that endogenous opioids depress chemosensitivity during hypoxia. In contrast, responsiveness to hypercapnia was reduced after the antagonist, implying that endogenous opioids may potentiate chemoreceptor sensitivity during hypercapnia. The results obtained using 'selective' agonists and antagonists provide evidence that depression of chemosensory discharge caused by injected opioids involves a delta type of opioid receptor within the cat carotid body. Endogenous opioids may modulate arterial chemoreceptor sensitivity to physiological stimuli such as hypoxia and hypercapnia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Demoliou-Mason C. Molecular properties of opioid receptors. Br Med Bull. 1983 Jan;39(1):37–45. doi: 10.1093/oxfordjournals.bmb.a071788. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Lillian A., Hazum E., Cuatrecasas P., Chang J. K. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): a potent and specific agonist for morphine (mu) receptors. Science. 1981 Apr 3;212(4490):75–77. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Gillan M. G., Kosterlitz H. W., McKnight A. T., Paterson S. J., Robson L. E. Selectivities of opioid peptide analogues as agonists and antagonists at the delta-receptor. Br J Pharmacol. 1984 Sep;83(1):271–279. doi: 10.1111/j.1476-5381.1984.tb10143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- Cotton R., Kosterlitz H. W., Paterson S. J., Rance M. J., Traynor J. R. The use of [3H]-[D-Pen2,D-Pen5]enkephalin as a highly selective ligand for the delta-binding site. Br J Pharmacol. 1985 Apr;84(4):927–932. doi: 10.1111/j.1476-5381.1985.tb17387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A., Zhu X. Z., Porreca F. Studies in vivo with ICI 174864 and [D-Pen2, D-Pen5]enkephalin. Neuropeptides. 1985 Feb;5(4-6):311–314. doi: 10.1016/0143-4179(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Dave J. R., Hotchkiss A. J., Affolter H. U. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984 Dec 13;312(5995):661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br J Pharmacol. 1982 Nov;77(3):461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz F., de Lourdes Guerrero M., Way E. L., Li C. H. Effect of beta-endorphin on calcium uptake in the brain. Science. 1979 Oct 5;206(4414):89–91. doi: 10.1126/science.39340. [DOI] [PubMed] [Google Scholar]
- Hansen J. T., Brokaw J., Christie D., Karasek M. Localization of enkephalin-like immunoreactivity in the cat carotid and aortic body chemoreceptors. Anat Rec. 1982 Jul;203(3):405–410. doi: 10.1002/ar.1092030310. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Fahrenkrug J., Nilsson G., Terenius L. Peptides in the cat carotid body (glomus caroticum): VIP-, enkephalin-, and substance P-like immunoreactivity. Acta Physiol Scand. 1979 Nov;107(3):279–281. doi: 10.1111/j.1748-1716.1979.tb06475.x. [DOI] [PubMed] [Google Scholar]
- Magnan J., Paterson S. J., Tavani A., Kosterlitz H. W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. A quantitative study of the effects of cholinergic drugs on carotid chemoreceptors in the cat. J Physiol. 1977 Dec;273(2):515–532. doi: 10.1113/jphysiol.1977.sp012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. S., Ribeiro J. A. Effects of beta-endorphin, vasoactive intestinal polypeptide and cholecystokinin octapeptide on cat carotid chemoreceptor activity. Q J Exp Physiol. 1981 Jul;66(3):273–284. doi: 10.1113/expphysiol.1981.sp002556. [DOI] [PubMed] [Google Scholar]
- McQueen D. S., Ribeiro J. A. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Br J Pharmacol. 1980;71(1):297–305. doi: 10.1111/j.1476-5381.1980.tb10939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti-Bloch L., Eyzaguirre C. Effects of methionine-enkephalin and substance P on the chemosensory discharge of the cat carotid body. Brain Res. 1985 Jul 15;338(2):297–307. doi: 10.1016/0006-8993(85)90160-x. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E., Jahr C. E. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980 Sep 4;287(5777):22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- North R. A. Opiates, opioid peptides and single neurones. Life Sci. 1979 Apr 23;24(17):1527–1545. doi: 10.1016/0024-3205(79)90014-6. [DOI] [PubMed] [Google Scholar]
- Paterson S. J., Robson L. E., Kosterlitz H. W. Classification of opioid receptors. Br Med Bull. 1983 Jan;39(1):31–36. doi: 10.1093/oxfordjournals.bmb.a071787. [DOI] [PubMed] [Google Scholar]
- Pokorski M., Lahiri S. Effects of naloxone on carotid body chemoreception and ventilation in the cat. J Appl Physiol Respir Environ Exerc Physiol. 1981 Dec;51(6):1533–1538. doi: 10.1152/jappl.1981.51.6.1533. [DOI] [PubMed] [Google Scholar]
- Shaw J. S., Miller L., Turnbull M. J., Gormley J. J., Morley J. S. Selective antagonists at the opiate delta-receptor. Life Sci. 1982 Sep 20;31(12-13):1259–1262. doi: 10.1016/0024-3205(82)90356-3. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Miller R. J. Opiates, second messengers and cell response. Br Med Bull. 1983 Jan;39(1):53–58. doi: 10.1093/oxfordjournals.bmb.a071791. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Pearse A. G., McGregor G. P., Bryant M. G., Bloom S. R., Emson P. C., Bisgard G. E., Will J. A. Enkephalin-, VIP- and substance P-like immunoreactivity in the carotid body. Nature. 1980 Mar 20;284(5753):269–271. doi: 10.1038/284269a0. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Egan T. M., North R. A. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982 Sep 2;299(5878):74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]


