Abstract
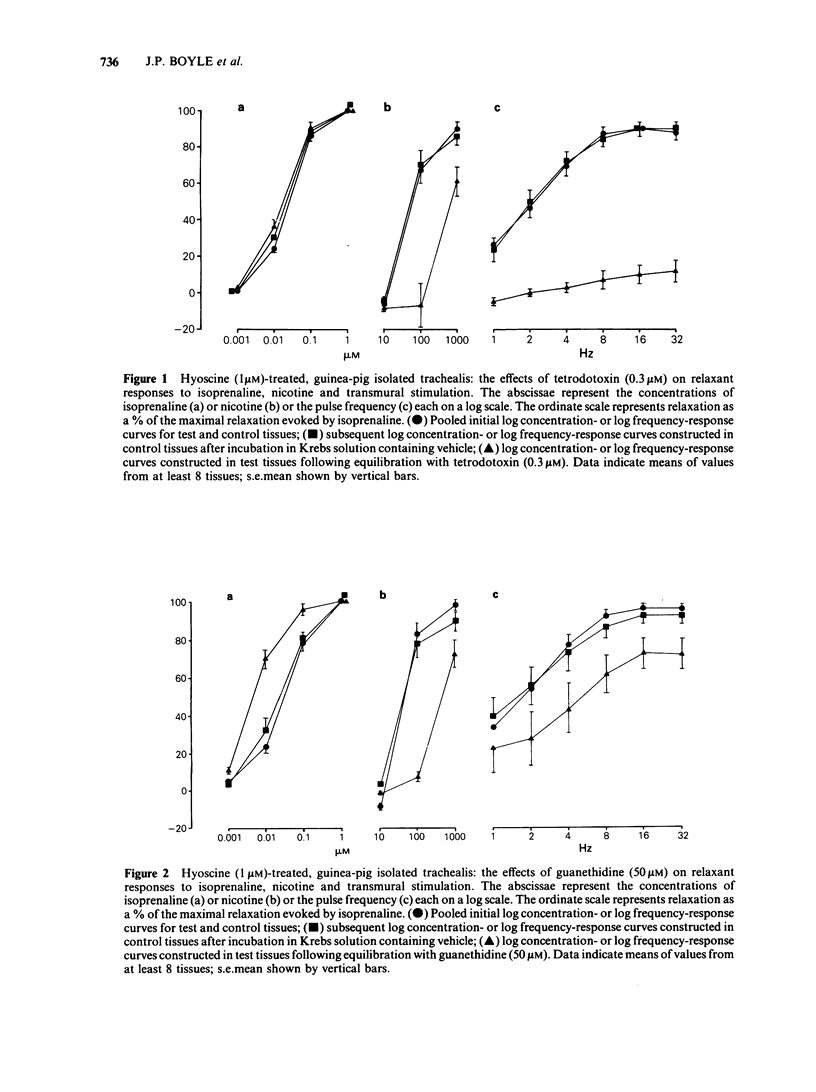
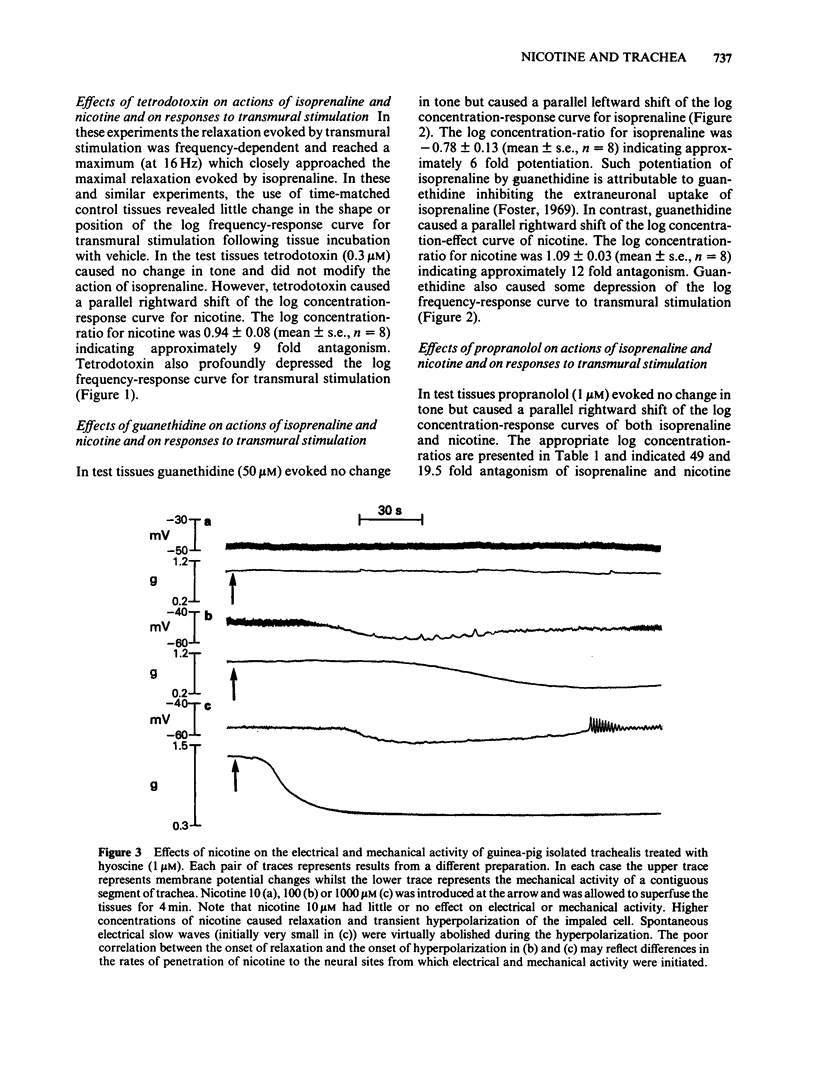
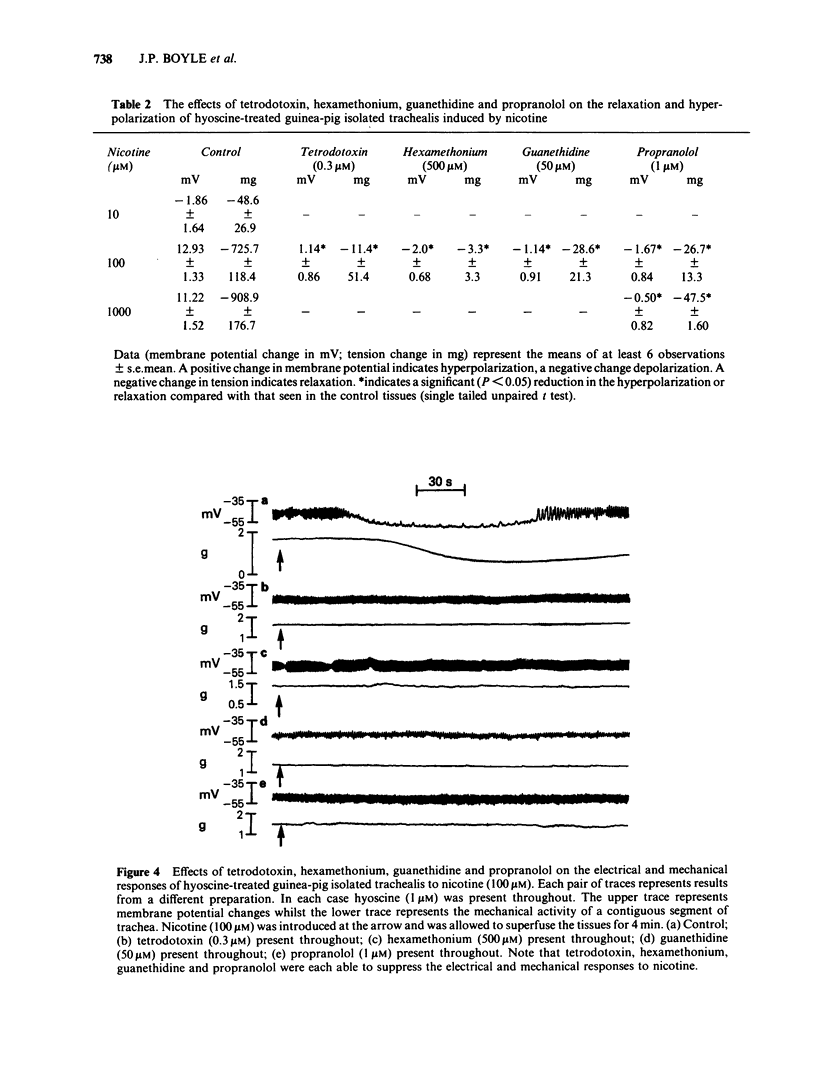
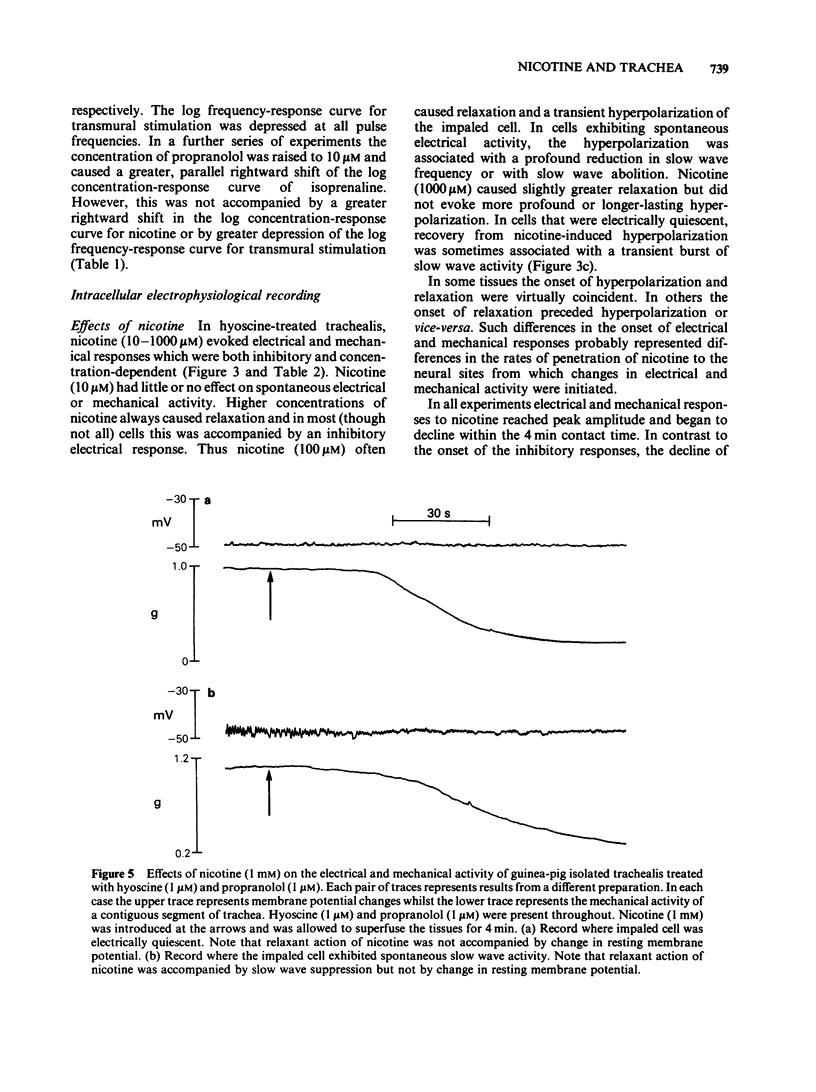
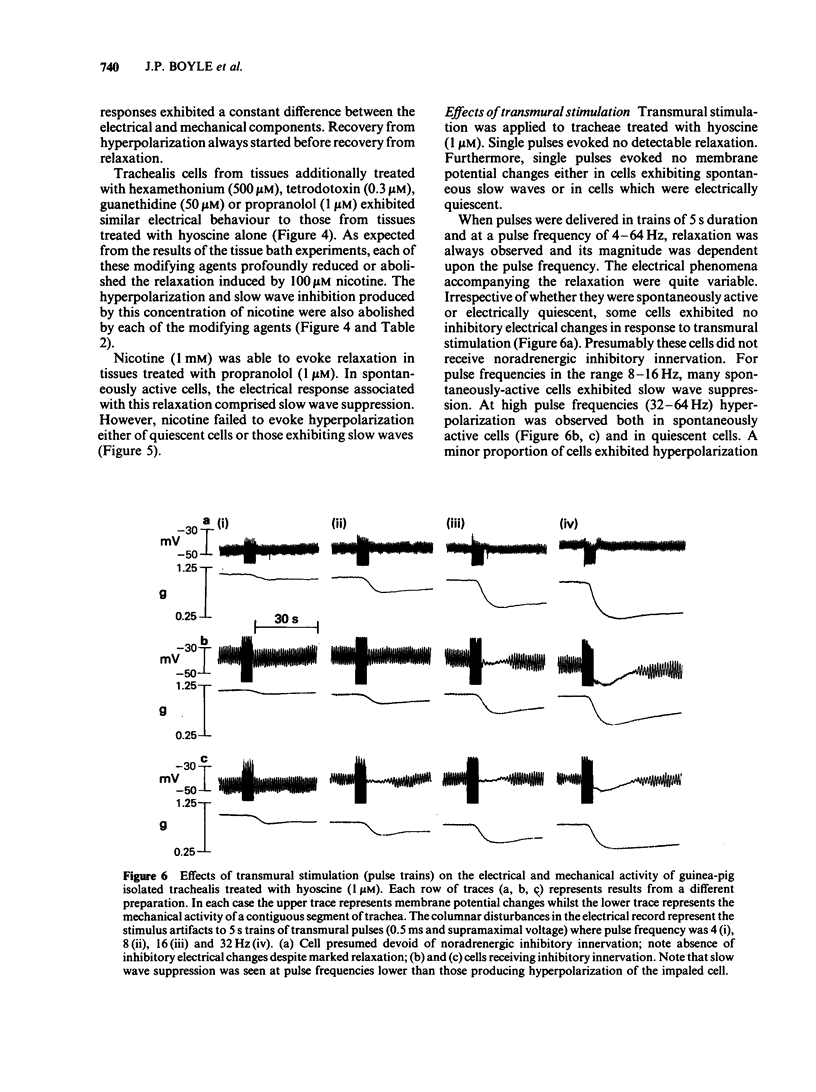
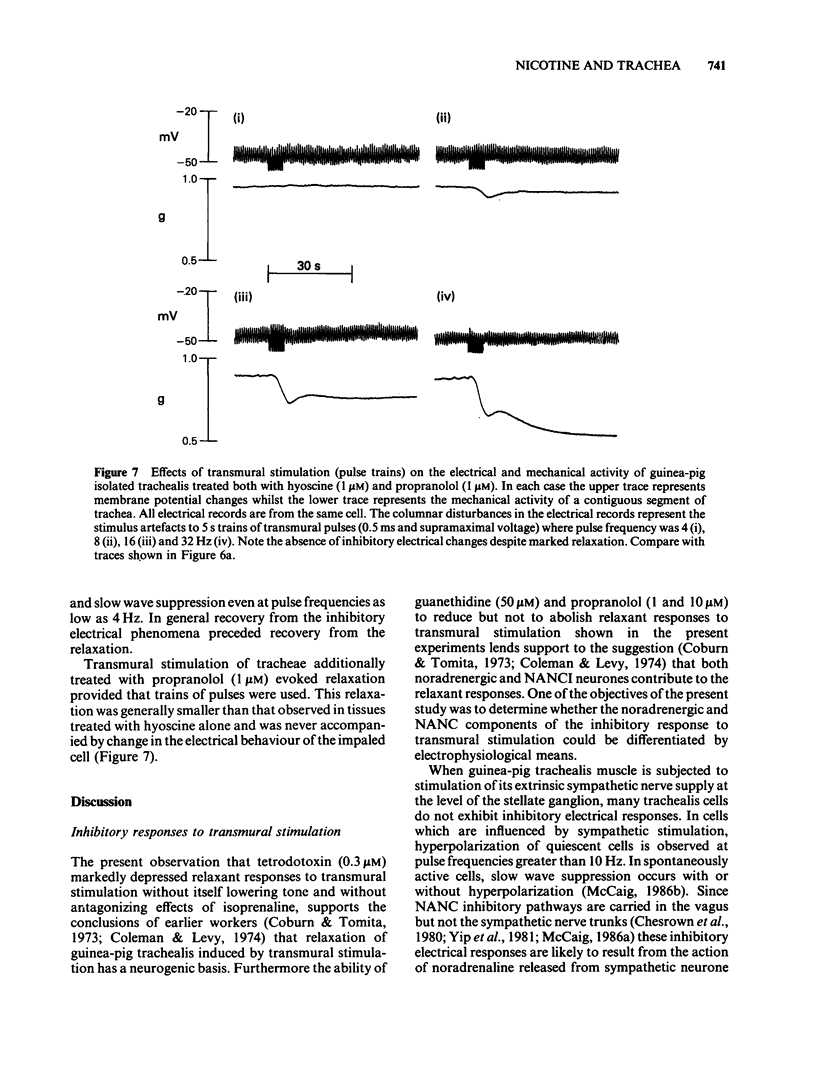
Guinea-pig isolated trachealis muscle treated with hyoscine (1 microM) exhibited mechanical tone which could be suppressed by transmural stimulation and, in a concentration-dependent manner, by nicotine (10-1000 microM). Hexamethonium (500 microM) did not itself cause tone changes, antagonized effects of nicotine but did not antagonize those of isoprenaline. Tetrodotoxin (0.3 microM) did not itself cause tone changes, did not modify the action of isoprenaline but antagonized the effects of nicotine and very markedly reduced responses to transmural electrical stimulation. Guanethidine (50 microM) did not itself cause tone changes, potentiated the action of isoprenaline, antagonized effects of nicotine and reduced responses to transmural electrical stimulation. Propranolol (1 microM) did not itself cause tone changes, antagonized effects of both isoprenaline and nicotine and reduced responses to transmural electrical stimulation. Propranolol (10 microM) caused greater antagonism of isoprenaline but did not further antagonize nicotine or further reduce responses to electrical stimulation. Intracellular electrophysiological recording from hyoscine-treated trachealis showed that 10 microM nicotine caused little or no mechanical or electrical change. Higher concentrations (100 microM and 1 mM) evoked relaxation which was often though not invariably accompanied by transient hyperpolarization and transient inhibition of electrical slow waves in the impaled cell. Hexamethonium (500 microM), tetrodotoxin (0.3 microM), guanethidine (50 microM) and propranolol (1 microM) each suppressed the electrical or mechanical changes evoked by nicotine (100 microM). However, nicotine (1 mM) tested in the presence of propranolol (1 microM), caused relaxation which could be accompanied by slow wave suppression but not by change in resting membrane potential. Transmural stimulation of hyoscine-treated trachea with single pulses of supramaximal voltage and 0.5 ms duration evoked neither relaxation nor membrane potential changes. Stimulation with similar pulses in trains of 5 s duration evoked relaxation which was dependent on pulse frequency. In many cells this relaxation was not accompanied by membrane potential change. In other cells suppression of slow waves occurred. At high pulse frequencies (greater than 16 Hz) this was generally accompanied by membrane hyperpolarization. In tissue treated with hyoscine and propranolol (both 1 microM), transmural stimulation with pulse trains as described above always evoked relaxation but no membrane potential changes were observed.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Beech D. J., Foster R. W., Morgan G. P., Small R. C. Electrophysiological and other aspects of the relaxant action of isoprenaline in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Dec;86(4):843–854. doi: 10.1111/j.1476-5381.1985.tb11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. R., Johnston C. F., Kirkpatrick C. T., Kirkpatrick M. C. The quest for the inhibitory neurotransmitter in bovine tracheal smooth muscle. Q J Exp Physiol. 1983 Jul;68(3):413–426. doi: 10.1113/expphysiol.1983.sp002735. [DOI] [PubMed] [Google Scholar]
- Chesrown S. E., Venugopalan C. S., Gold W. M., Drazen J. M. In vivo demonstration of nonadrenergic inhibitory innervation of the guinea pig trachea. J Clin Invest. 1980 Feb;65(2):314–320. doi: 10.1172/JCI109674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973 May;224(5):1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Levy G. P. A non-adrenergic inhibitory nervous pathway in guinea-pig trachea. Br J Pharmacol. 1974 Oct;52(2):167–174. doi: 10.1111/j.1476-5381.1974.tb09697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. S., Small R. C. Evidence of poor conduction of muscle excitation in the longitudinal axis of guinea-pig isolated trachea. Br J Pharmacol. 1983 May;79(1):75–83. doi: 10.1111/j.1476-5381.1983.tb10498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W. An uptake of radioactivity from (plus or minus)-3H-isoprenaline and its inhibition by drugs which potentiate the responses to (minus)-isoprenaline in the guinea-pig isolated trachea. Br J Pharmacol. 1969 Mar;35(3):418–427. doi: 10.1111/j.1476-5381.1969.tb08283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. W., Small R. C., Weston A. H. Evidence that the spasmogenic action of tetraethylammonium in guinea-pig trachealis is both direct and dependent on the cellular influx of calcium ion. Br J Pharmacol. 1983 May;79(1):255–263. doi: 10.1111/j.1476-5381.1983.tb10519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKINS D. F., PATON W. D. Responses of isolated bronchial muscle to ganglionically active drugs. J Physiol. 1958 Dec 4;144(2):193–219. doi: 10.1113/jphysiol.1958.sp006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Satake T., Takagi K., Tomita T. Effects of relaxants on electrical and mechanical activities in the guinea-pig tracheal muscle. Br J Pharmacol. 1986 Apr;87(4):665–671. doi: 10.1111/j.1476-5381.1986.tb14583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyes A. D., Barber P. Innervation of the trachealis muscle in the guinea-pig: a quantitative ultrastructural study. J Anat. 1980 Jun;130(Pt 4):789–800. [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Kannan M. S., Daniel E. E. Ultrastructural study of guinea pig tracheal smooth muscle and its innervation. Can J Physiol Pharmacol. 1980 Aug;58(8):974–983. doi: 10.1139/y80-148. [DOI] [PubMed] [Google Scholar]
- Krauss K. R., Carpenter D. O., Kopin I. J. Acetylcholine-induced release of norepinephrine in the presence of tetrodotoxin. J Pharmacol Exp Ther. 1970 Jun;173(2):416–421. [PubMed] [Google Scholar]
- McCaig D. J. Autonomic responses of the isolated, innervated trachea of the guinea-pig: interaction with autonomic drugs, histamine and 5-hydroxytryptamine. Br J Pharmacol. 1986 May;88(1):239–248. doi: 10.1111/j.1476-5381.1986.tb09492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig D. J. Electrophysiology of neuroeffector transmission in the isolated, innervated trachea of the guinea-pig. Br J Pharmacol. 1986 Dec;89(4):793–801. doi: 10.1111/j.1476-5381.1986.tb11184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Saar N. Histochemical localization of adrenergic nerves in the guinea-pig trachea. Br J Pharmacol. 1973 Apr;47(4):707–710. doi: 10.1111/j.1476-5381.1973.tb08197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano A. J., Rembish R. A. Negative chronotropic effects of McN A-343 and nicotine in isolated guinea-pig atria: insensitivity to blockade by tetrodotoxin. J Pharmacol Exp Ther. 1971 Apr;177(1):40–47. [PubMed] [Google Scholar]
- Yip P., Palombini B., Coburn R. F. Inhibitory innervation to the guinea pig trachealis muscle. J Appl Physiol Respir Environ Exerc Physiol. 1981 Feb;50(2):374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]


