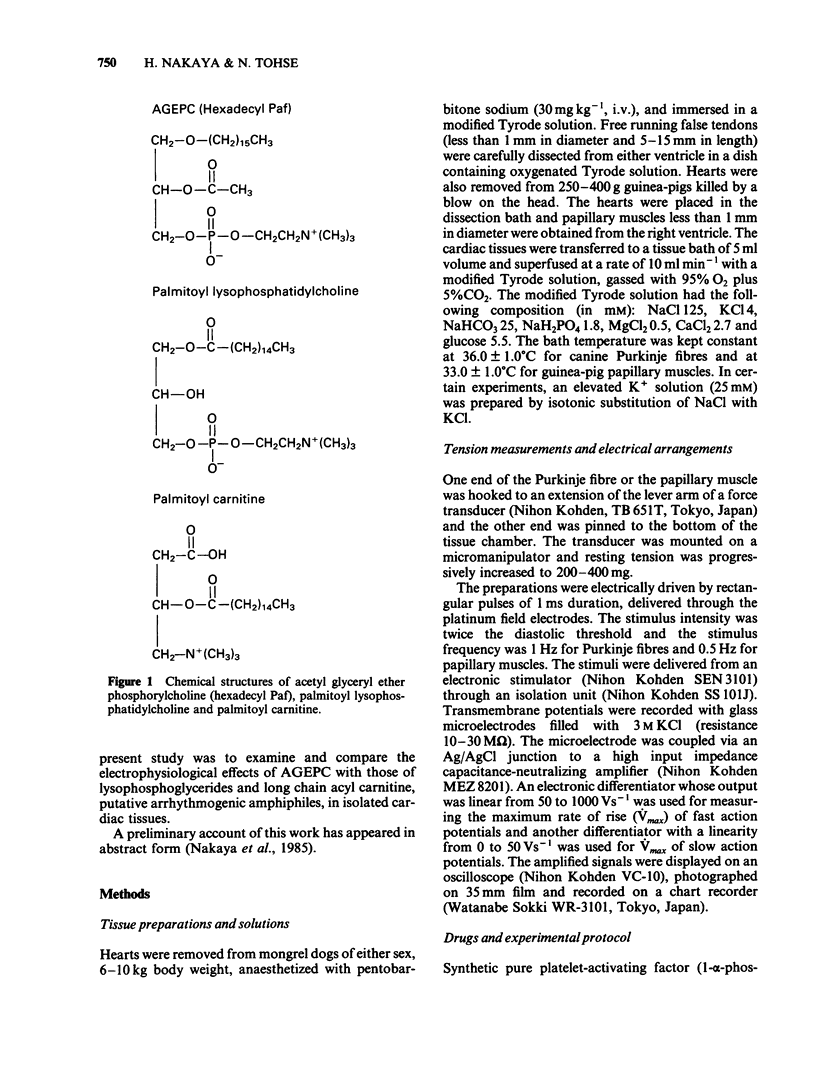
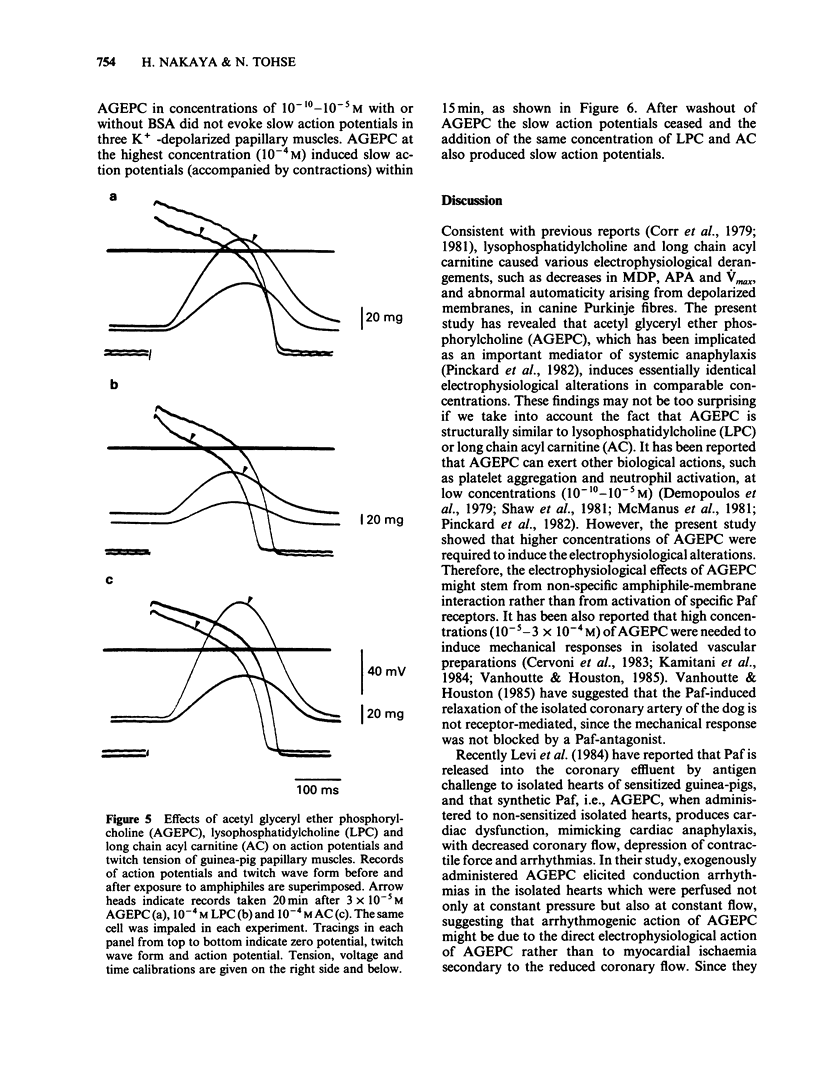
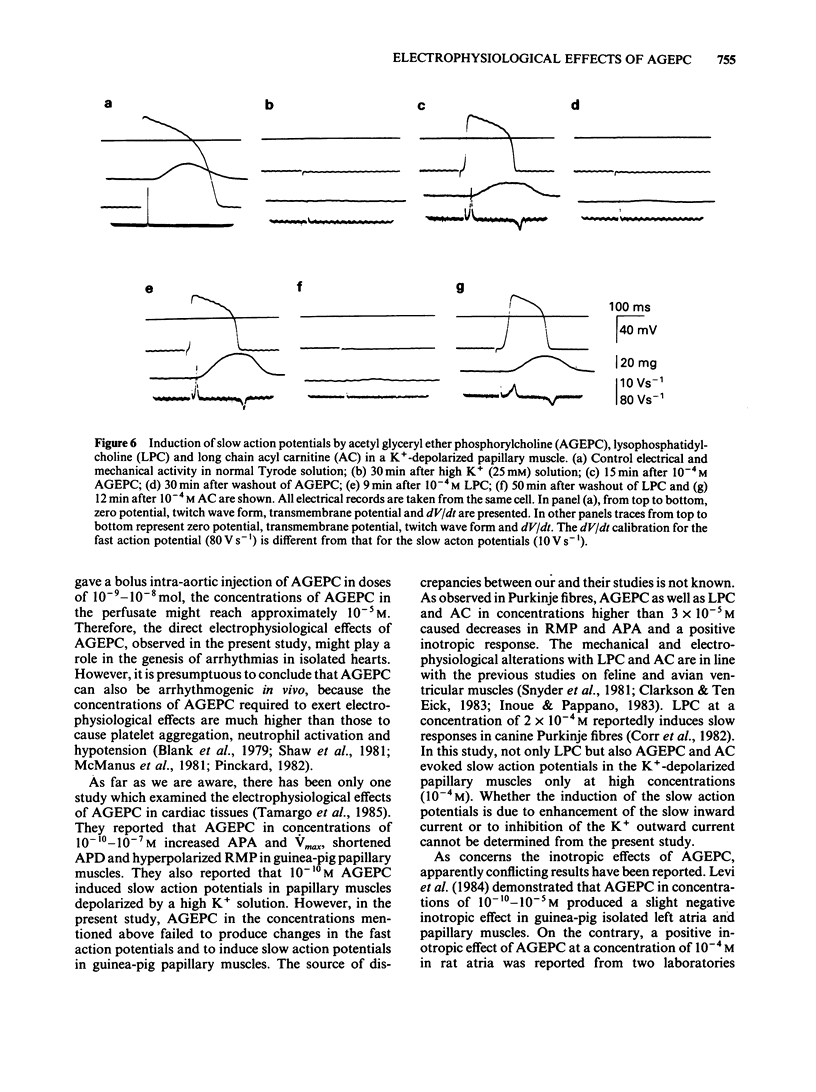
Abstract
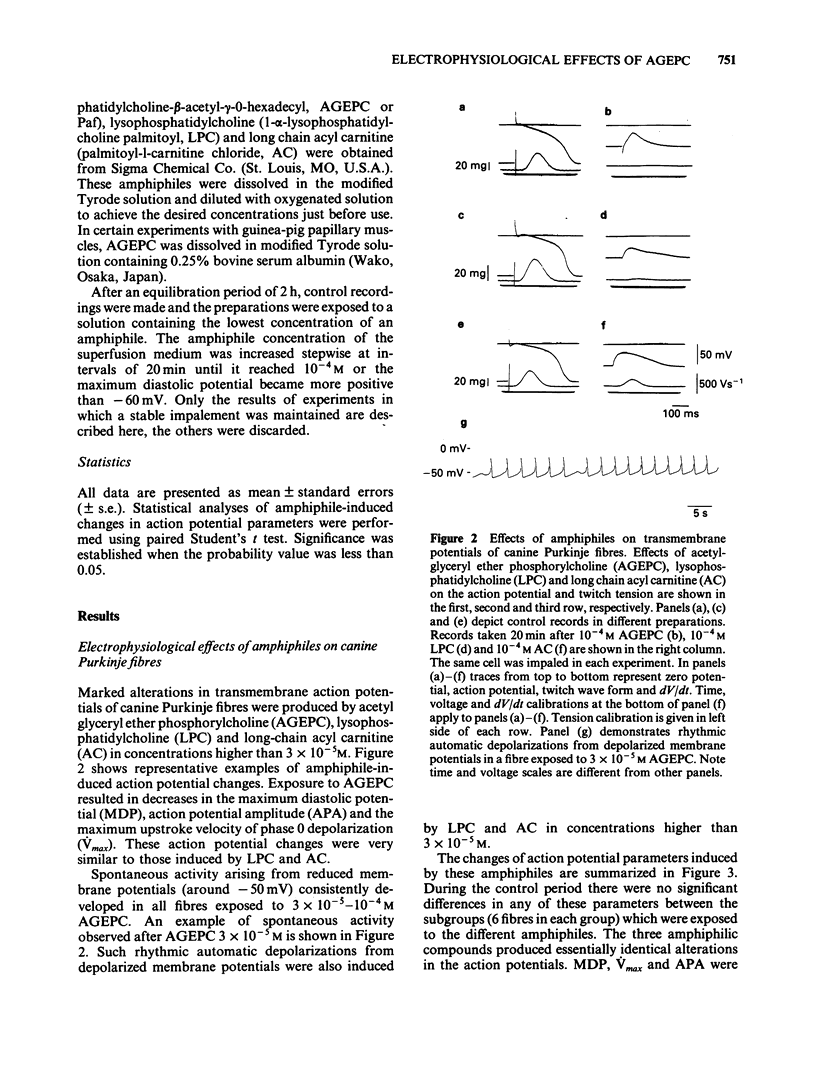
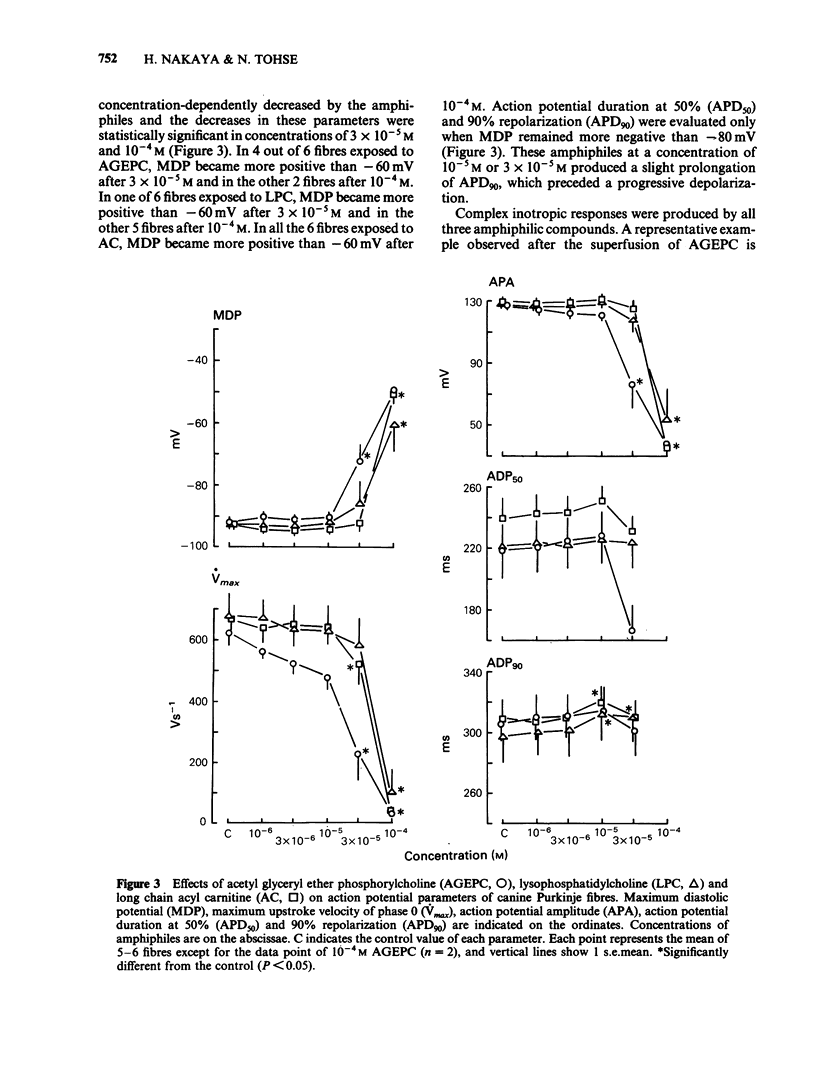
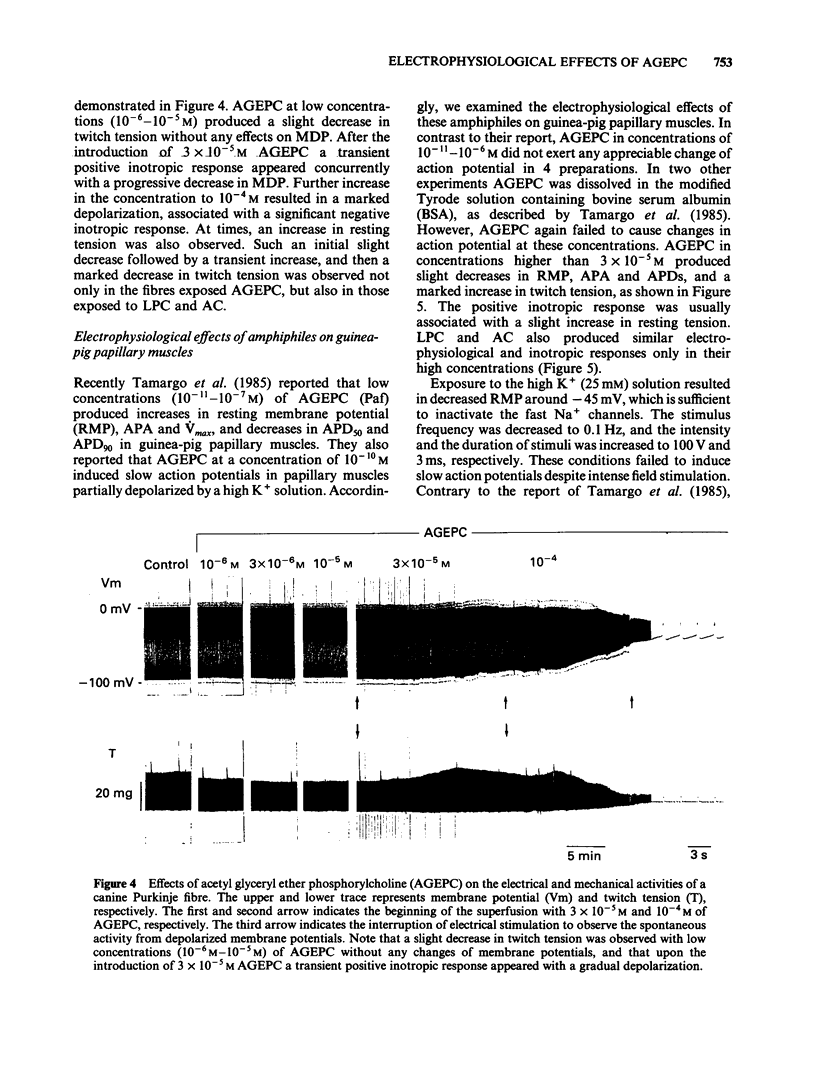
Electrophysiological effects of synthetic platelet activating factor, acetyl glyceryl ether phosphorylcholine (AGEPC), were examined and compared with those of lysophosphatidylcholine (LPC) and long chain acyl carnitine (AC) in canine Purkinje fibres and guinea-pig papillary muscles, by use of standard microelectrode techniques. In canine Purkinje fibres, AGEPC at concentrations higher than 3 X 10(-5)M, decreased maximum diastolic potential, action potential amplitude and the maximum upstroke velocity of phase 0. AGEPC also induced abnormal automaticity arising from depolarized membrane potentials. LPC and AC in concentrations higher than 3 X 10(-5)M also produced virtually identical electrophysiological alterations in Purkinje fibres. Although twitch tension was slightly decreased by low concentrations (10(-6)-10(-5)M) of these amphiphilic lipids, a transient positive inotropic response appeared at the beginning of a progressive depolarization after exposure to higher concentrations of the amphiphiles. In guinea-pig papillary muscles, AGEPC in concentrations higher than 3 X 10(-5)M produced slight decreases in resting membrane potential, action potential amplitude and action potential durations, concomitantly with a positive inotropic response. These electrophysiological and mechanical changes were also induced by LPC and AC at comparable concentrations. In guinea-pig papillary muscles depolarized with 25 mM [K+]0, AGEPC, LPC and AC all evoked slow action potentials at a concentration of 10(-4)M. It is concluded that in isolated cardiac tissues AGEPC exerts electrophysiological effects similar to those of LPC and AC only at high concentrations, and that the non-specific interaction of amphiphiles with sarcolemmal membrane may be responsible for the electrophysiological and mechanical effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Cervoni P., Herzlinger H. E., Lai F. M., Tanikella T. K. Aortic vascular and atrial responses to (+/-)-1-O-octadecyl-2-acetyl-glyceryl-3-phosphorylcholine. Br J Pharmacol. 1983 Jul;79(3):667–671. doi: 10.1111/j.1476-5381.1983.tb10003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C. W., Ten Eick R. E. On the mechanism of lysophosphatidylcholine-induced depolarization of cat ventricular myocardium. Circ Res. 1983 May;52(5):543–556. doi: 10.1161/01.res.52.5.543. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Cain M. E., Witkowski F. X., Price D. A., Sobel B. E. Potential arrhythmogenic electrophysiological derangements in canine Purkinje fibers induced by lysophosphoglycerides. Circ Res. 1979 Jun;44(6):822–832. doi: 10.1161/01.res.44.6.822. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Cain M. E., Crafford W. A., Jr, Gross R. W., Sobel B. E. Electrophysiological effects of amphiphiles on canine purkinje fibers. Implications for dysrhythmia secondary to ischemia. Circ Res. 1981 Aug;49(2):354–363. doi: 10.1161/01.res.49.2.354. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Lee B. I., Gross R. W., Keim C. R., Sobel B. E. Pathophysiological concentrations of lysophosphatides and the slow response. Am J Physiol. 1982 Aug;243(2):H187–H195. doi: 10.1152/ajpheart.1982.243.2.H187. [DOI] [PubMed] [Google Scholar]
- Danon Y. L., Seeger R. C., Maidman J. E. Fetal neural antigens on human neuroblastoma cells. J Immunol. 1980 Jun;124(6):2925–2929. [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Findlay S. R., Lichtenstein L. M., Hanahan D. J., Pinckard R. N. Contraction of guinea pig ileal smooth muscle by acetyl glyceryl ether phosphorylcholine. Am J Physiol. 1981 Sep;241(3):C130–C133. doi: 10.1152/ajpcell.1981.241.3.C130. [DOI] [PubMed] [Google Scholar]
- Fink K. L., Gross R. W. Modulation of canine myocardial sarcolemmal membrane fluidity by amphiphilic compounds. Circ Res. 1984 Nov;55(5):585–594. doi: 10.1161/01.res.55.5.585. [DOI] [PubMed] [Google Scholar]
- Halonen M., Palmer J. D., Lohman I. C., McManus L. M., Pinckard R. N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis. 1980 Dec;122(6):915–924. doi: 10.1164/arrd.1980.122.6.915. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Inoue D., Pappano A. J. L-palmitylcarnitine and calcium ions act similarly on excitatory ionic currents in avian ventricular muscle. Circ Res. 1983 Jun;52(6):625–634. doi: 10.1161/01.res.52.6.625. [DOI] [PubMed] [Google Scholar]
- Kamitani T., Katamoto M., Tatsumi M., Katsuta K., Ono T., Kikuchi H., Kumada S. Mechanism(s) of the hypotensive effect of synthetic 1-O-octadecyl-2-O-acetyl-glycero-3-phosphorylcholine. Eur J Pharmacol. 1984 Mar 2;98(3-4):357–366. doi: 10.1016/0014-2999(84)90284-x. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J., Nellis S., Neely J. R. Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ Res. 1978 Oct;43(4):652–661. doi: 10.1161/01.res.43.4.652. [DOI] [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Pinckard R. N. Human platelet stimulation by acetyl glyceryl ether phosphorylcholine. J Clin Invest. 1981 Mar;67(3):903–906. doi: 10.1172/JCI110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Langer G. A., Rich T. L. Charged amphiphiles regulate heart contractility and sarcolemma-Ca2+ interactions. Am J Physiol. 1985 Jan;248(1 Pt 2):H147–H150. doi: 10.1152/ajpheart.1985.248.1.H147. [DOI] [PubMed] [Google Scholar]
- Sedlis S. P., Corr P. B., Sobel B. E., Ahumada G. G. Lysophosphatidyl choline potentiates Ca2+ accumulation in rat cardiac myocytes. Am J Physiol. 1983 Jan;244(1):H32–H38. doi: 10.1152/ajpheart.1983.244.1.H32. [DOI] [PubMed] [Google Scholar]
- Shaikh N. A., Downar E. Time course of changes in porcine myocardial phospholipid levels during ischemia. A reassessment of the lysolipid hypothesis. Circ Res. 1981 Aug;49(2):316–325. doi: 10.1161/01.res.49.2.316. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Snyder D. W., Crafford W. A., Jr, Glashow J. L., Rankin D., Sobel B. E., Corr P. B. Lysophosphoglycerides in ischemic myocardium effluents and potentiation of their arrhythmogenic effects. Am J Physiol. 1981 Nov;241(5):H700–H707. doi: 10.1152/ajpheart.1981.241.5.H700. [DOI] [PubMed] [Google Scholar]
- Tamargo J., Tejerina T., Delgado C., Barrigon S. Electrophysiological effects of platelet-activating factor (PAF-acether) in guinea-pig papillary muscles. Eur J Pharmacol. 1985 Feb 26;109(2):219–227. doi: 10.1016/0014-2999(85)90423-6. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Houston D. S. Platelets, endothelium, and vasospasm. Circulation. 1985 Oct;72(4):728–734. doi: 10.1161/01.cir.72.4.728. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]


