Abstract
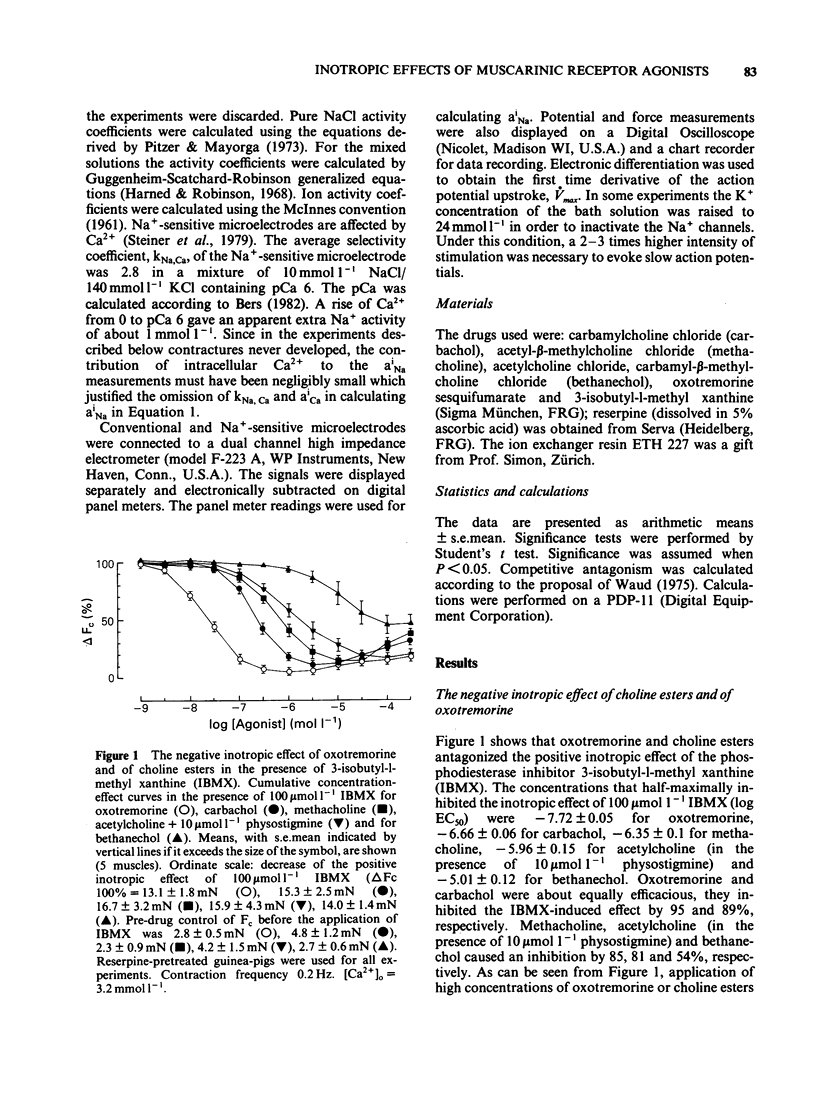
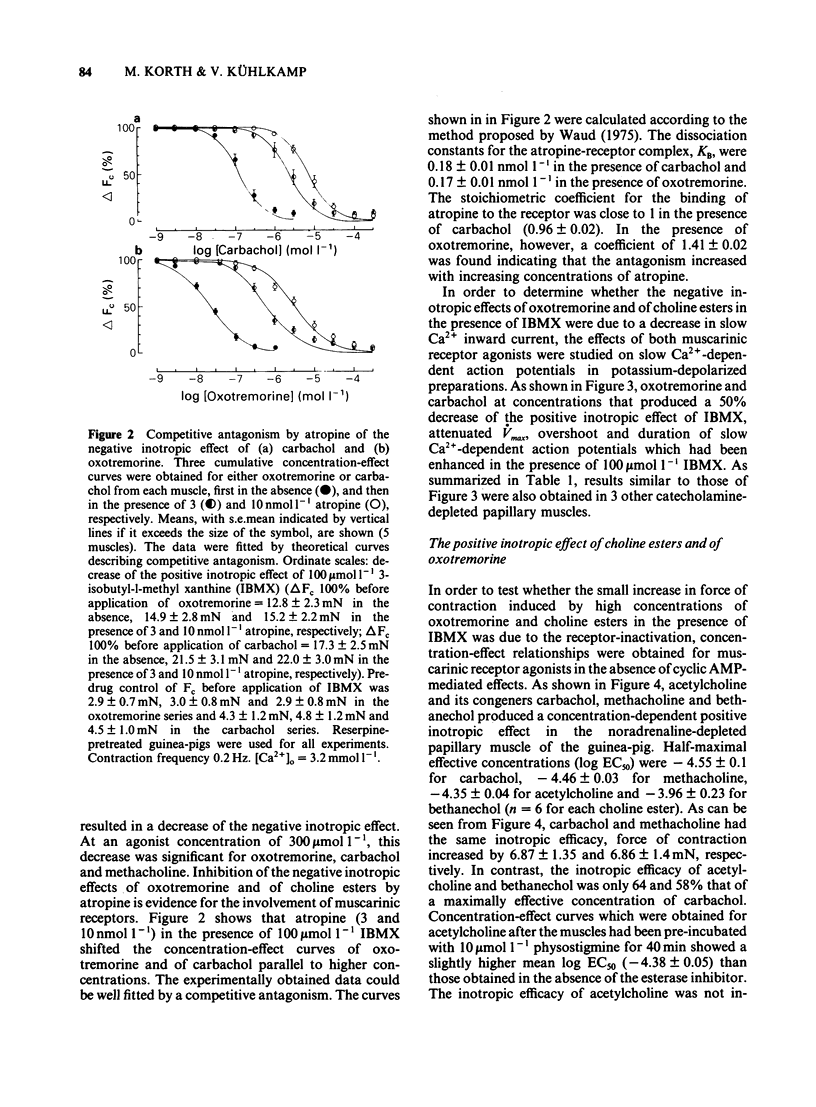
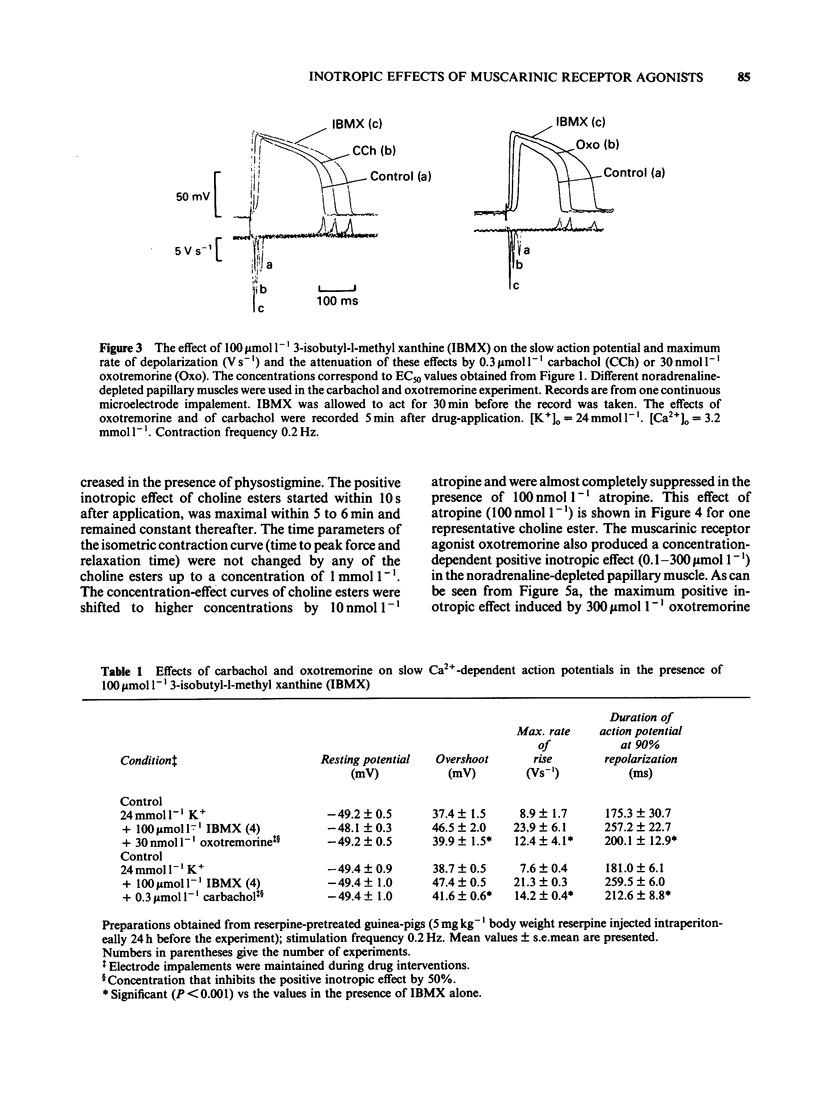
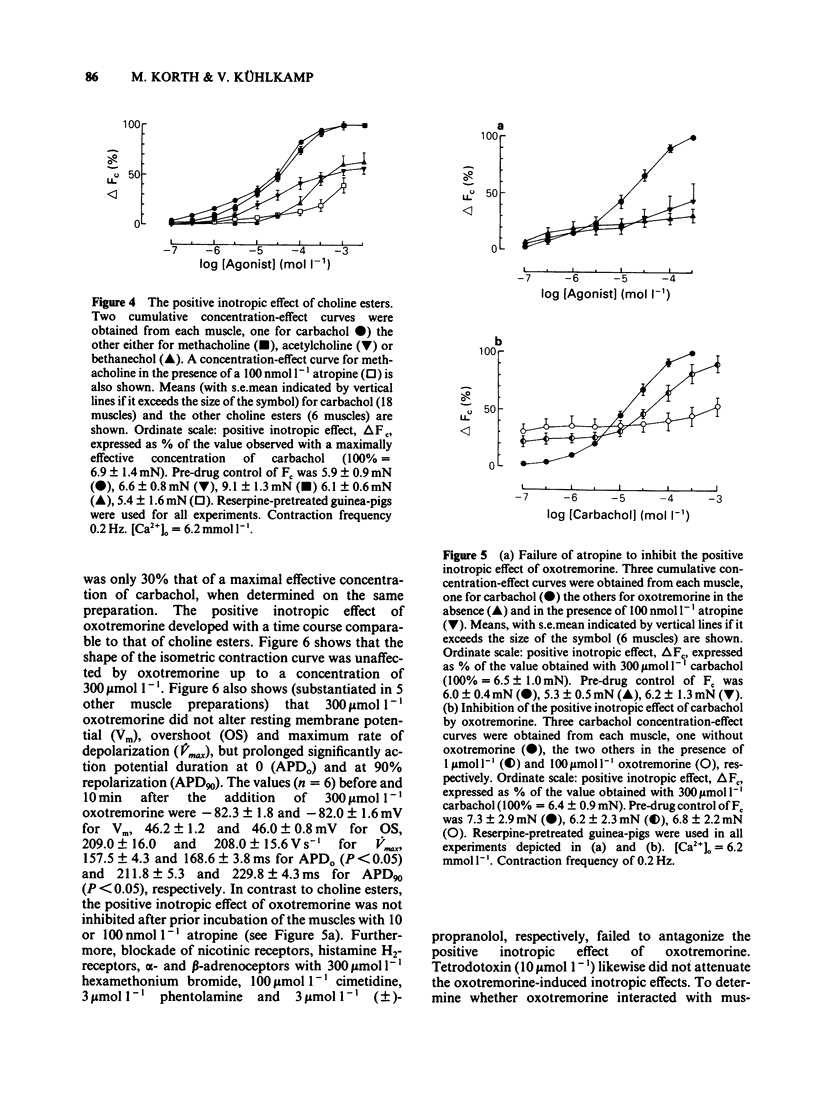
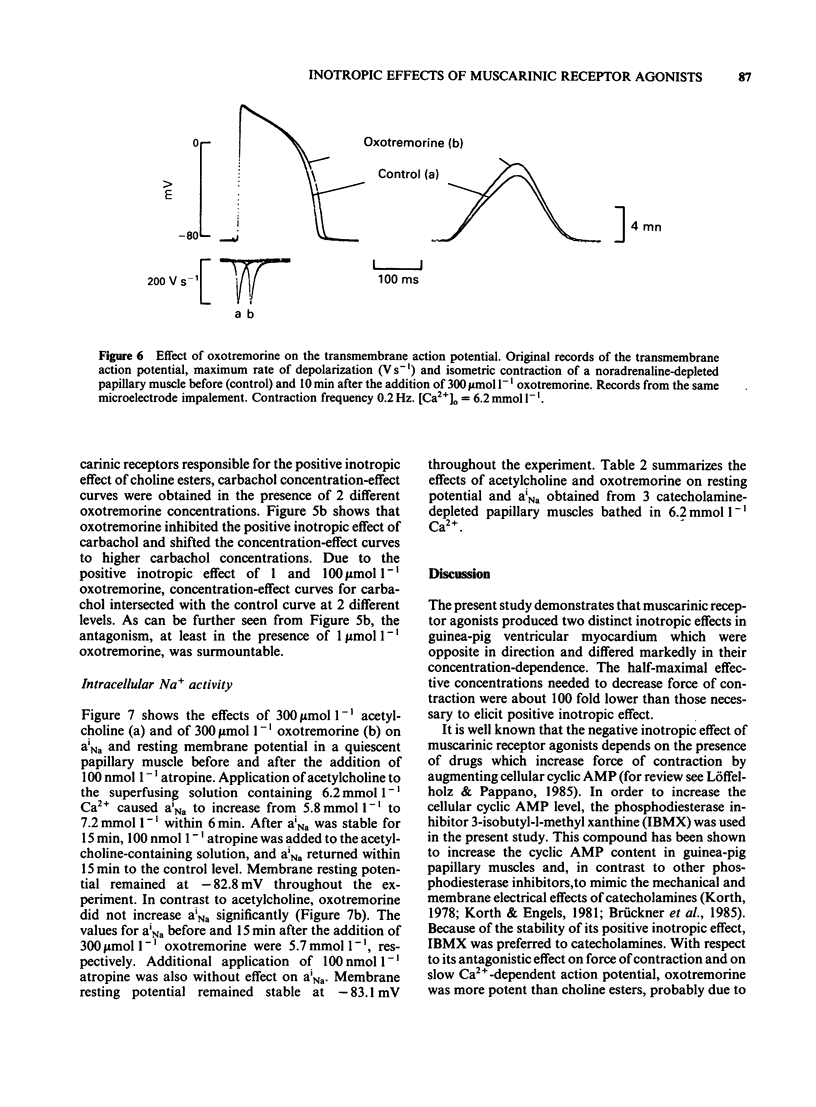
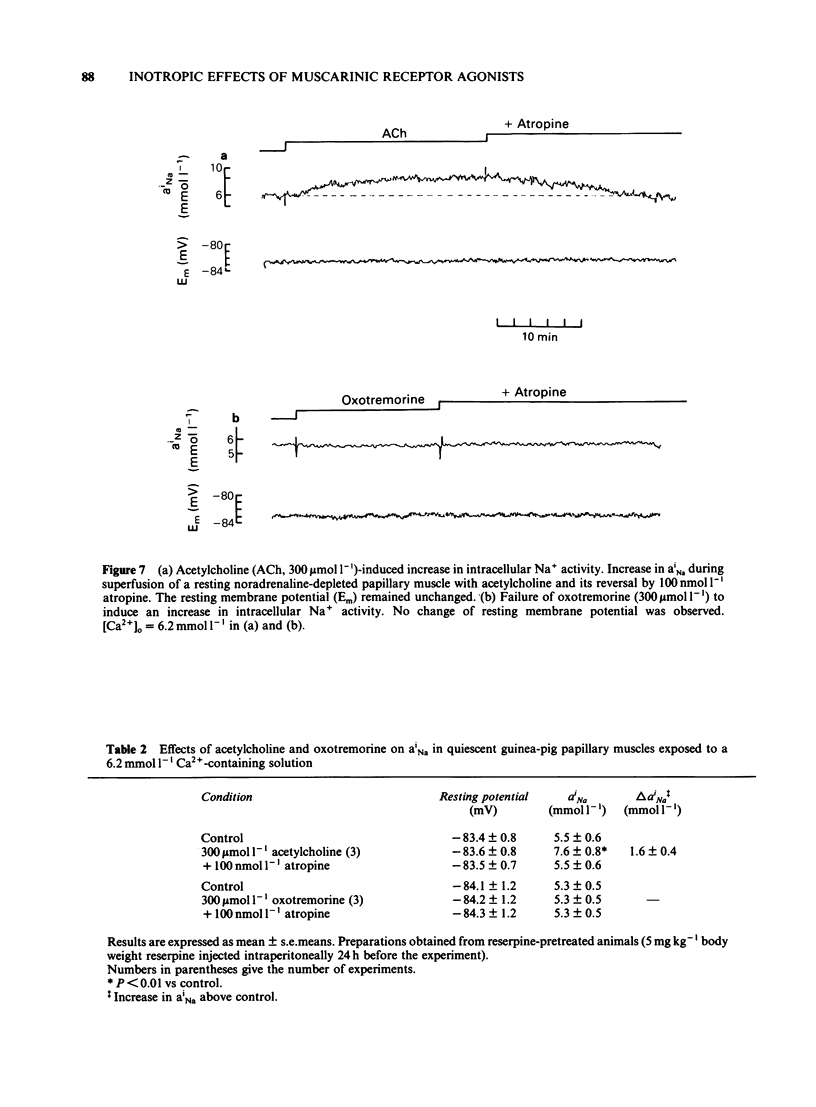
The concentration-dependence of the negative and positive inotropic effect of choline esters and of oxotremorine was studied in isometrically contracting papillary muscles of the guinea-pig. The preparations were obtained from reserpine-pretreated animals and were electrically driven at a frequency of 0.2 Hz. In the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methyl xanthine (IBMX, 100 mumol l-1), choline esters and oxotremorine produced concentration-dependent negative inotropic effects. Oxotremorine exhibited the highest negative inotropic potency (with a half-maximal effective concentration, EC50, of 20 nmol l-1) followed by carbachol (139 nmol l-1), methacholine (490 nmol l-1), acetylcholine in the presence of 10 mumol l-1 physostigmine (1.36 mumol l-1) and bethanechol (10 mumol l-1). Atropine was a competitive antagonist of the negative inotropic effects. Carbachol and oxotremorine decreased Vmax, overshoot and duration of slow Ca2+-dependent action potentials which had been elicited in the presence of 100 mumol l-1 IBMX. Choline esters produced a concentration-dependent positive inotropic effect. With an EC50 of 32 mumol l-1, carbachol was the most potent compound, followed by methacholine (35 mumol l-1), acetylcholine in the presence of 10 mumol l-1 physostigmine (46 mumol l-1) and bethanechol (142 mumol l-1). Compared to carbachol and methacholine which increased force by 100% of control, the increase induced by acetylcholine and bethanechol was only 64 and 58%, respectively. Atropine shifted the concentration-effect curves of all choline esters to higher concentrations. Choline esters caused intracellular Na+ activity to increase in the quiescent papillary muscle. This effect was reversed by atropine. Oxotremorine produced a small concentration-dependent positive inotropic effect (about 30% of the maximal effect of carbachol) which was resistant to atropine. Oxotremorine was a potent inhibitor of the positive inotropic effect of choline esters, and did not cause an increase in intracellular Na+ activity in the quiescent papillary muscle. The results show that muscarinic receptors of the ventricular myocardium mediate two inotropic effects, which are opposite in direction and differ in their concentration-dependence by a factor of 100. Although agonists differentiate between both inotropic effects, it is unknown whether the receptors involved represent receptor states or separate receptor subpopulations. The negative inotropic effect of choline esters and of oxotremorine can be best explained by adenylate cyclase inhibition.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Brown S. L. Agonists differentiate muscarinic receptors that inhibit cyclic AMP formation from those that stimulate phosphoinositide metabolism. J Biol Chem. 1984 Mar 25;259(6):3777–3781. [PubMed] [Google Scholar]
- Brown S. L., Brown J. H. Muscarinic stimulation of phosphatidylinositol metabolism in atria. Mol Pharmacol. 1983 Nov;24(3):351–356. [PubMed] [Google Scholar]
- Brückner R., Gramann S., Nose M., Schmitz W., Scholz H. Isoprenaline-like effects of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine on mechanical, biochemical and electrophysiological parameters in the mammalian heart. Experientia. 1985 Jun 15;41(6):732–734. doi: 10.1007/BF02012570. [DOI] [PubMed] [Google Scholar]
- Delhaye M., De Smet J. M., Taton G., De Neef P., Camus J. C., Fontaine J., Waelbroeck M., Robberecht P., Christophe J. A comparison between muscarinic receptor occupancy, adenylate cyclase inhibition, and inotropic response in human heart. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):170–175. doi: 10.1007/BF00506197. [DOI] [PubMed] [Google Scholar]
- George W. J., Wilkerson R. D., Kadowitz P. J. Influence of acetylcholine on contractile force and cyclic nucleotide levels in the isolated perfused rat heart. J Pharmacol Exp Ther. 1973 Jan;184(1):228–235. [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Winegrad S. Cholinergic regulation of calcium sensitivity in cardiac muscle. J Mol Cell Cardiol. 1983 Apr;15(4):277–280. doi: 10.1016/0022-2828(83)90282-1. [DOI] [PubMed] [Google Scholar]
- Korth M. Effects of several phosphodiesterase-inhibitors on guinea-pig myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1978 Mar;302(1):77–86. doi: 10.1007/BF00586601. [DOI] [PubMed] [Google Scholar]
- Korth M., Kühlkamp V. Muscarinic receptor-mediated increase of intracellular Na+-ion activity and force of contraction. Pflugers Arch. 1985 Mar;403(3):266–272. doi: 10.1007/BF00583598. [DOI] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- McMahon K. K., Hosey M. M. Agonist interactions with cardiac muscarinic receptors. Effects of Mg2+, guanine nucleotides, and monovalent cations. Mol Pharmacol. 1985 Nov;28(5):400–409. [PubMed] [Google Scholar]
- Quist E. E. Evidence for a carbachol stimulated phosphatidylinositol effect in heart. Biochem Pharmacol. 1982 Oct 1;31(19):3130–3133. doi: 10.1016/0006-2952(82)90095-8. [DOI] [PubMed] [Google Scholar]
- Reiter M. Die Wertbestimmung inotrop wirkender Arzneimittel am isolierten Papillarmuskel. Arzneimittelforschung. 1967 Oct;17(10):1249–1253. [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Di Virgilio F., Pozzan T. Inositol 1,4,5-trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature. 1985 Jul 25;316(6026):347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]


