Abstract
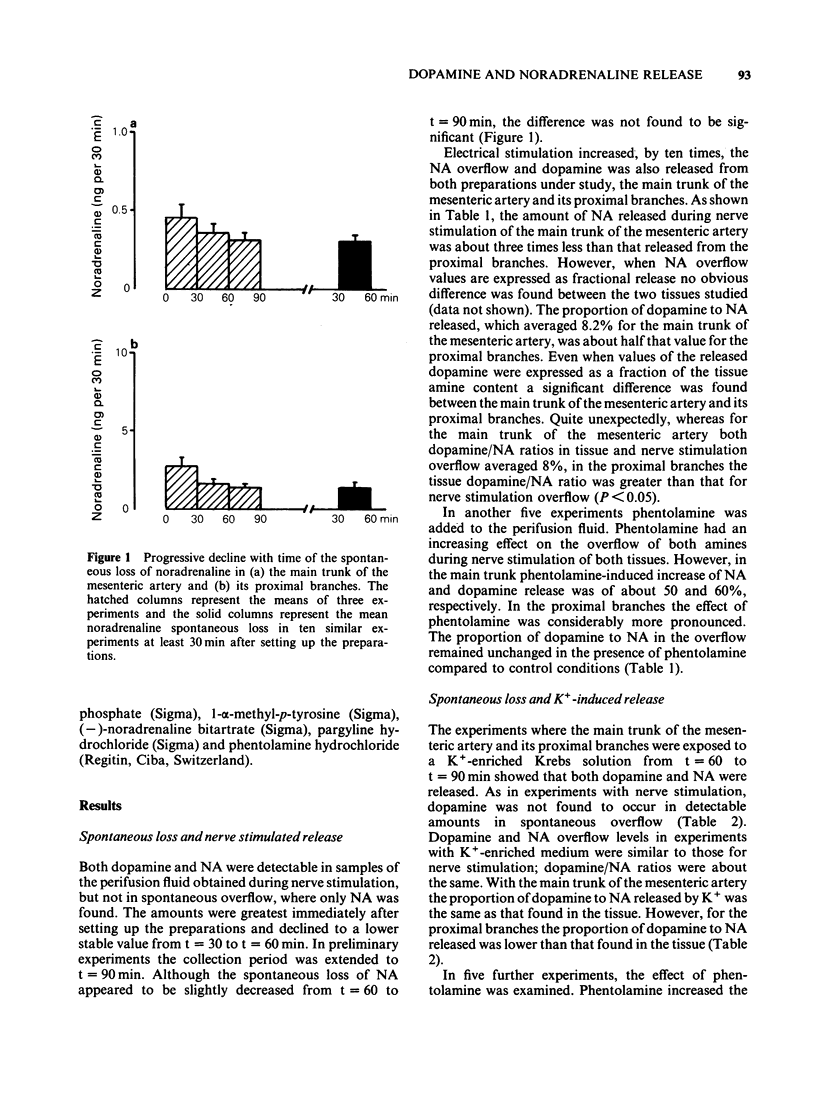
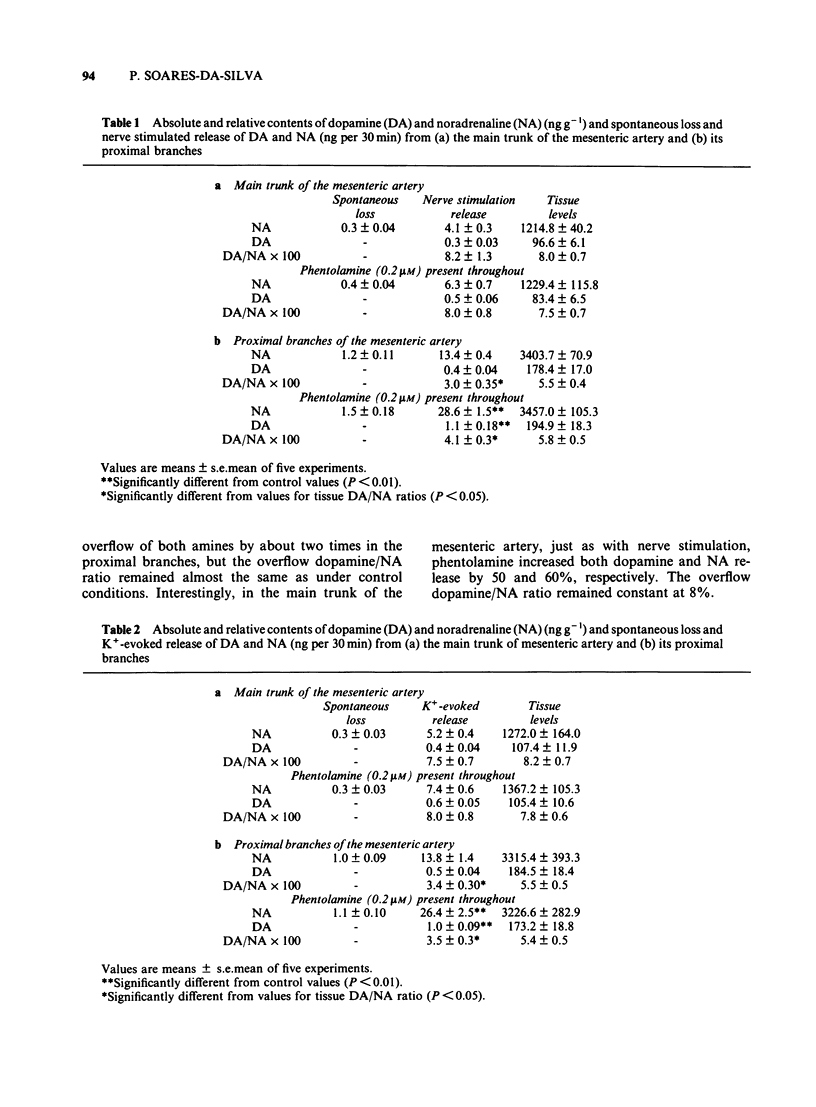
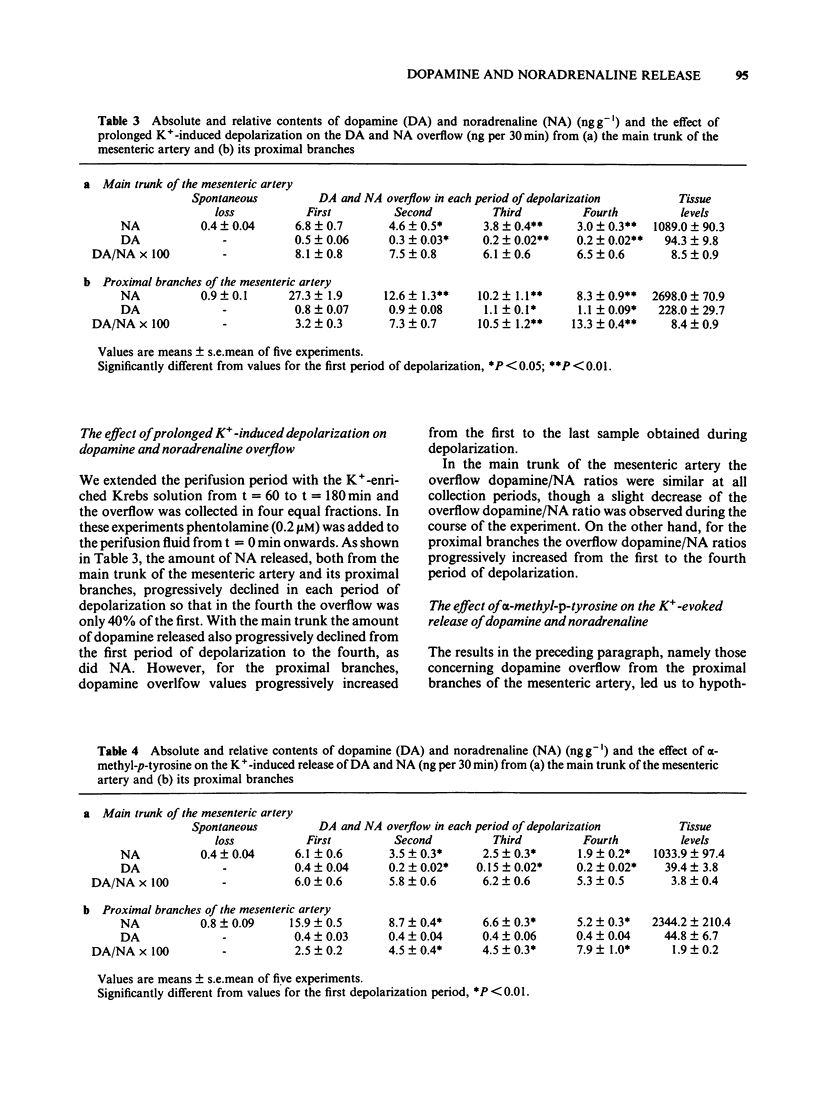
The release of dopamine and noradrenaline (NA), from the main trunk of the mesenteric artery and its proximal branches elicited by electrical nerve stimulation and K+, has been measured by using high pressure liquid chromatography with electrochemical detection. Both stimuli released dopamine and NA. With the main trunk of the mesenteric artery, dopamine represented 8% of the NA tissue content; the dopamine/NA ratio in the catecholamine overflow caused by nerve stimulation or K+-induced depolarization also averaged 8%. For the proximal branches the tissue dopamine/NA ratio was significantly greater than that observed to occur in the overflow caused by nerve stimulation and K+. When the perifusion with a K+-enriched medium was extended to 120 min the amount of NA released from both the main trunk and the proximal branches progressively declined. The same pattern of release was observed for dopamine in the main trunk, whereas for the proximal branches dopamine overflow did not decline throughout the perifusion period. The addition of alpha-methyl-p-tyrosine did not change the pattern of amine overflow. Our interpretation of these results is that both dopamine and NA are derived from the same sympathetic neurone. In the proximal branches of the mesenteric artery dopamine and NA appear to be in two different storage structures, whereas in the main trunk both dopamine and NA are located in only one storage structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Dopamine as a postganglionic autonomic neurotransmitter. Neuroscience. 1982 Jan;7(1):1–8. doi: 10.1016/0306-4522(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Bell C., Gillespie J. S. Dopamine and noradrenaline levels in peripheral tissues of several mammalian species. J Neurochem. 1981 Feb;36(2):703–706. doi: 10.1111/j.1471-4159.1981.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Bell C., Gillespie J. S., Macrae I. M. Release of noradrenaline and dopamine by nerve stimulation in the guinea-pig and rat vas deferens. Br J Pharmacol. 1984 Mar;81(3):563–569. doi: 10.1111/j.1476-5381.1984.tb10110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramona M. M., Soares-da-Silva P. The effects of chemical sympathectomy on dopamine, noradrenaline and adrenaline content in some peripheral tissues. Br J Pharmacol. 1985 Oct;86(2):351–356. doi: 10.1111/j.1476-5381.1985.tb08903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianutsos G., Moore K. E. Epinephrine contents of sympathetic ganglia and brain regions of spontaneously hypertensive rats of different ages. Proc Soc Exp Biol Med. 1978 May;158(1):45–49. doi: 10.3181/00379727-158-40136. [DOI] [PubMed] [Google Scholar]
- Gordon M. B., Moore T. J., Dluhy R. G., Williams G. H. Dopaminergic modulation of aldosterone responsiveness to angiotensin II with changes in sodium intake. J Clin Endocrinol Metab. 1983 Feb;56(2):340–345. doi: 10.1210/jcem-56-2-340. [DOI] [PubMed] [Google Scholar]
- Grobecker H., Saavedra J. M., Weise V. K. Biosynthetic enzyme activities and catecholamines in adrenal glands of genetic and experimental hypertensive rats. Circ Res. 1982 May;50(5):742–746. doi: 10.1161/01.res.50.5.742. [DOI] [PubMed] [Google Scholar]
- Klein R. L., Harden T. K. Newly synthesized norepinephrine accumulation in the readily releasable pool of a purified adrenergic vesicle fraction. Life Sci. 1975 Jan 15;16(2):315–322. doi: 10.1016/0024-3205(75)90030-2. [DOI] [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968 Jun;161(2):271–278. [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schömig A., Dart A. M., Dietz R., Mayer E., Kübler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: Locally mediated release. Circ Res. 1984 Nov;55(5):689–701. doi: 10.1161/01.res.55.5.689. [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva P., Davidson R. Effects of 6-hydroxydopamine on dopamine and noradrenaline content in dog blood vessels and heart. Evidence for a noradrenaline-independent dopamine pool. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):253–257. doi: 10.1007/BF00501876. [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva P. Evidence for a non-precursor dopamine pool in noradrenergic neurones of the dog mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jul;333(3):219–223. doi: 10.1007/BF00512932. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Weiner N. Regulation of norepinephrine biosynthesis. Annu Rev Pharmacol. 1970;10:273–290. doi: 10.1146/annurev.pa.10.040170.001421. [DOI] [PubMed] [Google Scholar]


