Abstract
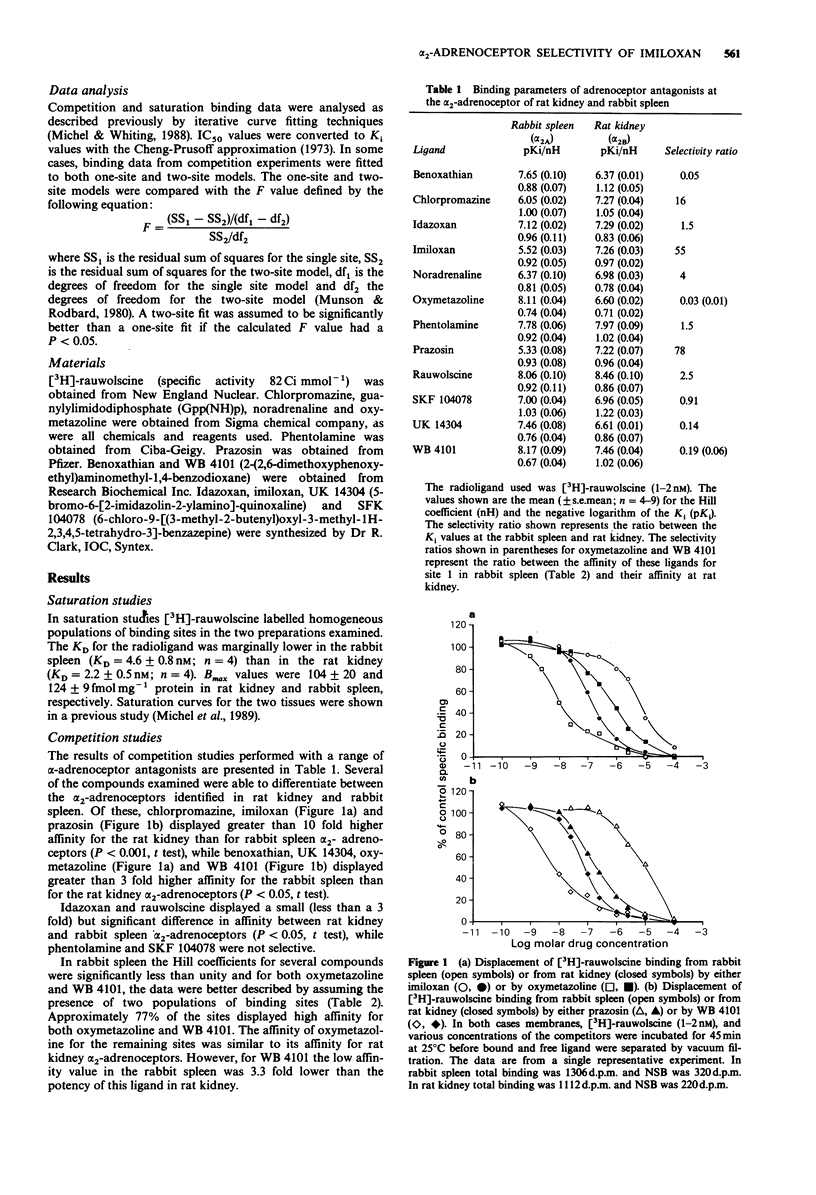
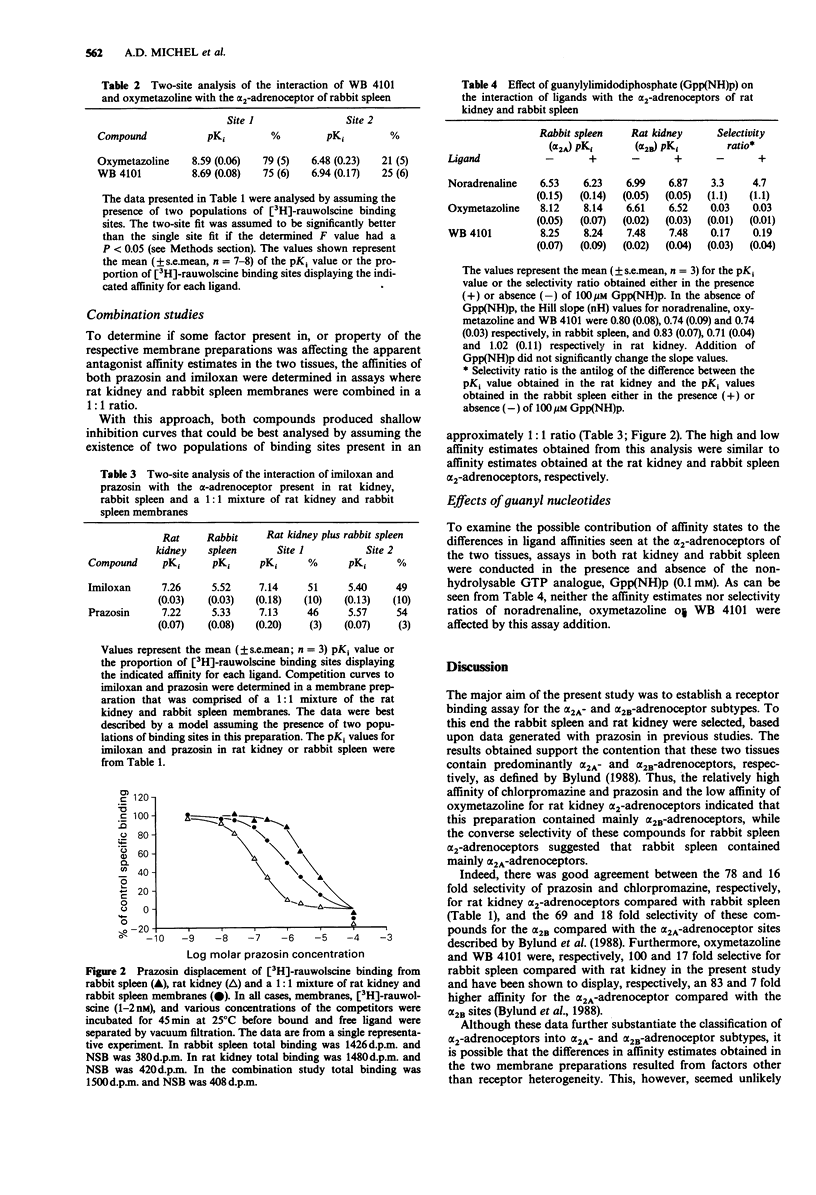
1. The alpha 2-adrenoceptor binding sites of rabbit spleen and rat kidney, labelled with [3H]-rauwolscine, were characterized using a range of subtype selective ligands. 2. In rabbit spleen, the alpha-2-adrenoceptor binding sites displayed high affinity for oxymetazoline and WB 4101 and low affinity for prazosin and chlorpromazine suggesting the presence of an alpha 2A subtype. 3. There was evidence for heterogeneity of the alpha 2-adrenoceptor binding sites present in rabbit spleen. The results obtained with oxymetazoline and WB 4101 indicated that at least 75% of the [3H]-rauwolscine binding sites in this preparation displayed a pharmacology consistent with the presence of an alpha 2A subtype. 4. In rat kidney, the alpha 2-adrenoceptor binding sites displayed high affinity for prazosin and chlorpromazine and low affinity for oxymetazoline and WB 4101 suggesting the presence of an alpha 2B subtype. 5. The inclusion of guanylylimidodiphosphate (Gpp(NH)p, 0.1 mM) did not modify the pharmacology of the alpha 2-adrenoceptor binding sites present in the two preparations. Furthermore, when the two membrane preparations were combined, the resultant pharmacology was still consistent with the presence of two receptors that retained the characteristics of the alpha 2A and alpha 2B subtypes. 6. Imiloxan was identified as a selective alpha 2B ligand while benoxathian displayed a high degree of selectivity for the alpha 2A-adrenoceptor binding site. The selectivity of imiloxan for the alpha 2B-adrenoceptor binding site, coupled with its specificity for alpha 2-adrenoceptors, should make it a valuable tool in the classification of alpha 2-adrenoceptor subtypes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyajian C. L., Leslie F. M. Pharmacological evidence for alpha-2 adrenoceptor heterogeneity: differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987 Jun;241(3):1092–1098. [PubMed] [Google Scholar]
- Broadhurst A. M., Alexander B. S., Wood M. D. Heterogeneous 3H-rauwolscine binding sites in rat cortex: two alpha 2-adrenoceptor subtypes or an additional non-adrenergic interaction? Life Sci. 1988;43(1):83–92. doi: 10.1016/0024-3205(88)90240-8. [DOI] [PubMed] [Google Scholar]
- Bylund D. B. Heterogeneity of alpha-2 adrenergic receptors. Pharmacol Biochem Behav. 1985 May;22(5):835–843. doi: 10.1016/0091-3057(85)90536-2. [DOI] [PubMed] [Google Scholar]
- Bylund D. B., Ray-Prenger C., Murphy T. J. Alpha-2A and alpha-2B adrenergic receptor subtypes: antagonist binding in tissues and cell lines containing only one subtype. J Pharmacol Exp Ther. 1988 May;245(2):600–607. [PubMed] [Google Scholar]
- Bylund D. B. Subtypes of alpha 2-adrenoceptors: pharmacological and molecular biological evidence converge. Trends Pharmacol Sci. 1988 Oct;9(10):356–361. doi: 10.1016/0165-6147(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cheung Y. D., Barnett D. B., Nahorski S. R. [3H]Rauwolscine and [3H]yohimbine binding to rat cerebral and human platelet membranes: possible heterogeneity of alpha 2-adrenoceptors. Eur J Pharmacol. 1982 Oct 15;84(1-2):79–85. doi: 10.1016/0014-2999(82)90159-5. [DOI] [PubMed] [Google Scholar]
- Coupry I., Podevin R. A., Dausse J. P., Parini A. Evidence for imidazoline binding sites in basolateral membranes from rabbit kidney. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1055–1060. doi: 10.1016/s0006-291x(87)80177-8. [DOI] [PubMed] [Google Scholar]
- Dickinson K. E., McKernan R. M., Miles C. M., Leys K. S., Sever P. S. Heterogeneity of mammalian alpha 2-adrenoceptors delineated by [3H]yohimbine binding. Eur J Pharmacol. 1986 Jan 29;120(3):285–293. doi: 10.1016/0014-2999(86)90469-3. [DOI] [PubMed] [Google Scholar]
- Diop L., Dausse J. P., Meyer P. Specific binding of [3H]rauwolscine to alpha 2-adrenoceptors in rat cerebral cortex: comparison between crude and synaptosomal plasma membranes. J Neurochem. 1983 Sep;41(3):710–715. doi: 10.1111/j.1471-4159.1983.tb04798.x. [DOI] [PubMed] [Google Scholar]
- Kawahara R. S., Bylund D. B. Solubilization and characterization of putative alpha-2 adrenergic isoceptors from the human platelet and the rat cerebral cortex. J Pharmacol Exp Ther. 1985 Jun;233(3):603–610. [PubMed] [Google Scholar]
- Lattimer N., Rhodes K. F. A difference in the affinity of some selective alpha 2-adrenoceptor antagonists when compared on isolated vasa deferentia of rat and rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):278–281. doi: 10.1007/BF00501880. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Brasili L., Giardinà D., Pigini M., Strappaghetti G. 2-[[[2-(2,6-Dimethoxyphenoxy)ethyl]amino]-methyl] -1,4-benzoxathian: a new antagonist with high potency and selectivity toward alpha 1-adrenoreceptors. J Med Chem. 1984 Dec;27(12):1535–1536. doi: 10.1021/jm00378a001. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Loury D. N., Whiting R. L. Differences between the alpha 2-adrenoceptor in rat submaxillary gland and the alpha 2A-and alpha 2B-adrenoceptor subtypes. Br J Pharmacol. 1989 Nov;98(3):890–897. doi: 10.1111/j.1476-5381.1989.tb14618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies. Eur J Pharmacol. 1988 Jan 5;145(1):61–66. doi: 10.1016/0014-2999(88)90349-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Neylon C. B., Summers R. J. [3H]-rauwolscine binding to alpha 2-adrenoceptors in the mammalian kidney: apparent receptor heterogeneity between species. Br J Pharmacol. 1985 Jun;85(2):349–359. doi: 10.1111/j.1476-5381.1985.tb08868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. W., Kobilka T. S., Yang-Feng T. L., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Sulpizio A. C., Nichols A. J., DeMarinis R. M., Hieble J. P. Pharmacologic differentiation between pre- and postjunctional alpha 2-adrenoceptors by SK&F 104078. Naunyn Schmiedebergs Arch Pharmacol. 1987 Oct;336(4):415–418. doi: 10.1007/BF00164875. [DOI] [PubMed] [Google Scholar]
- Summers R. J., Barnett D. B., Nahorski S. R. The characteristics of adrenoceptors in homogenates of human cerebral cortex labelled by (3H)-rauwolscine. Life Sci. 1983 Sep 12;33(11):1105–1112. doi: 10.1016/0024-3205(83)90667-7. [DOI] [PubMed] [Google Scholar]
- Turner J. T., Pierce D. L., Bylund D. B. Alpha-2 adrenergic regulation of norepinephrine release in the rat submandibular gland as measured by HPLC-EC. Life Sci. 1984 Sep 24;35(13):1385–1394. doi: 10.1016/0024-3205(84)90396-5. [DOI] [PubMed] [Google Scholar]


