Abstract
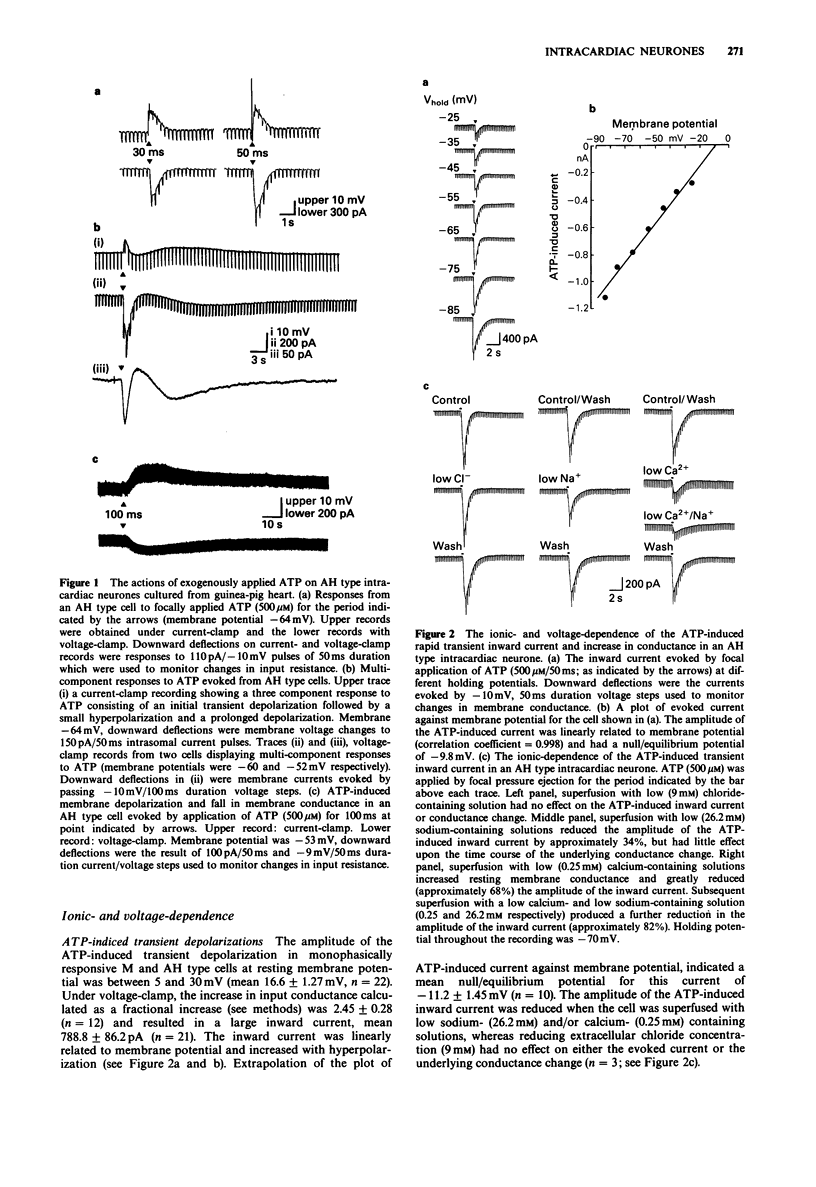
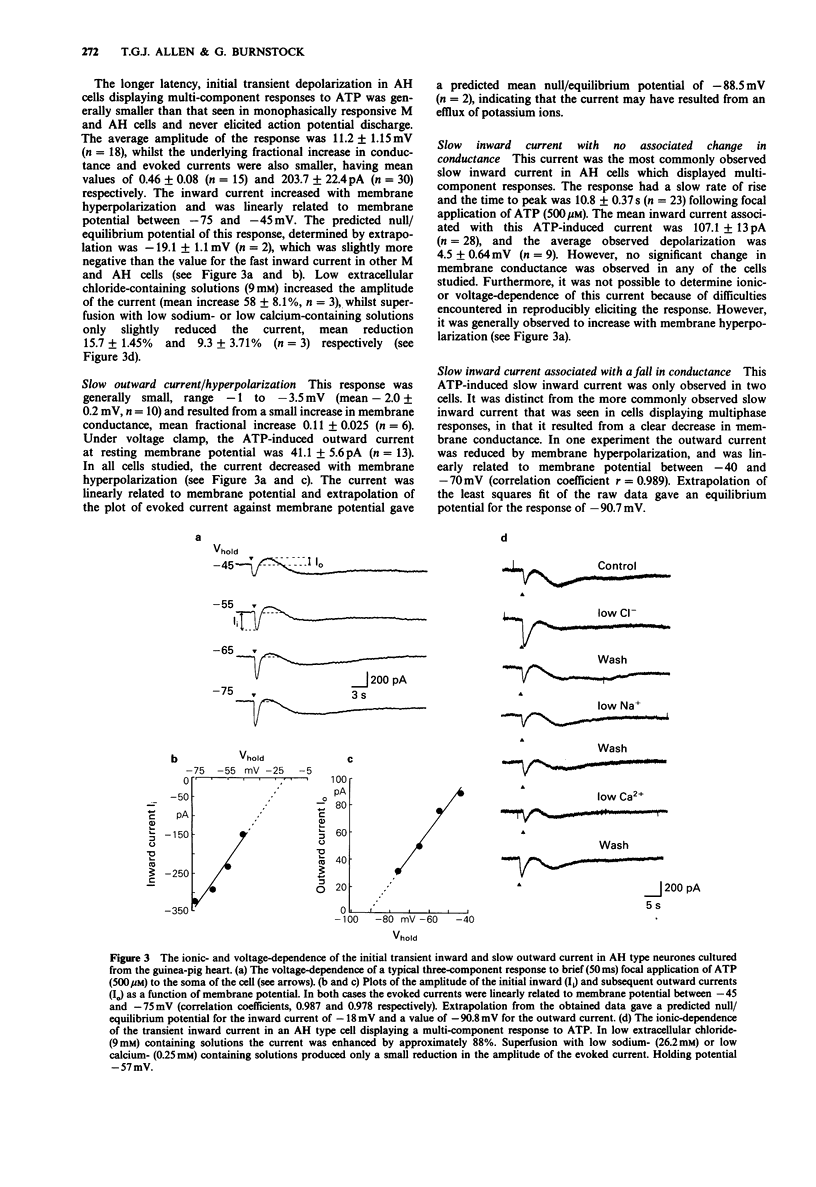
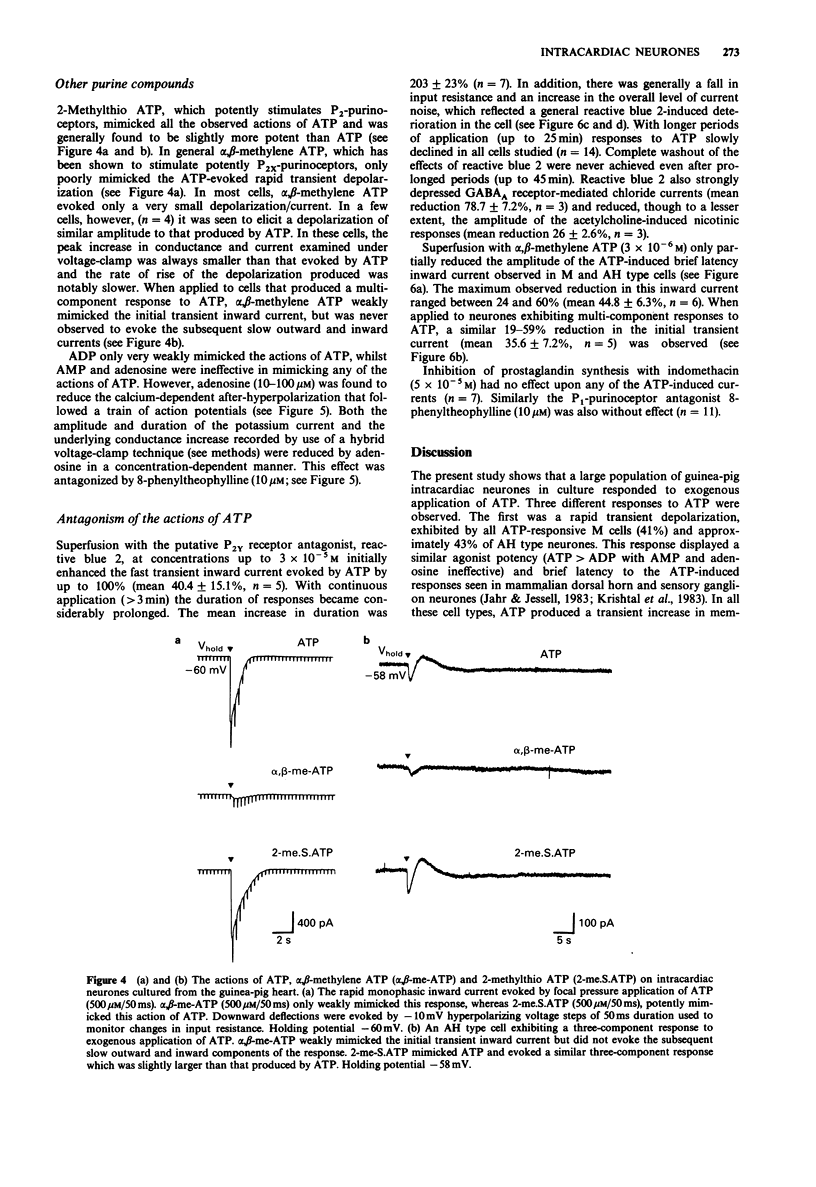
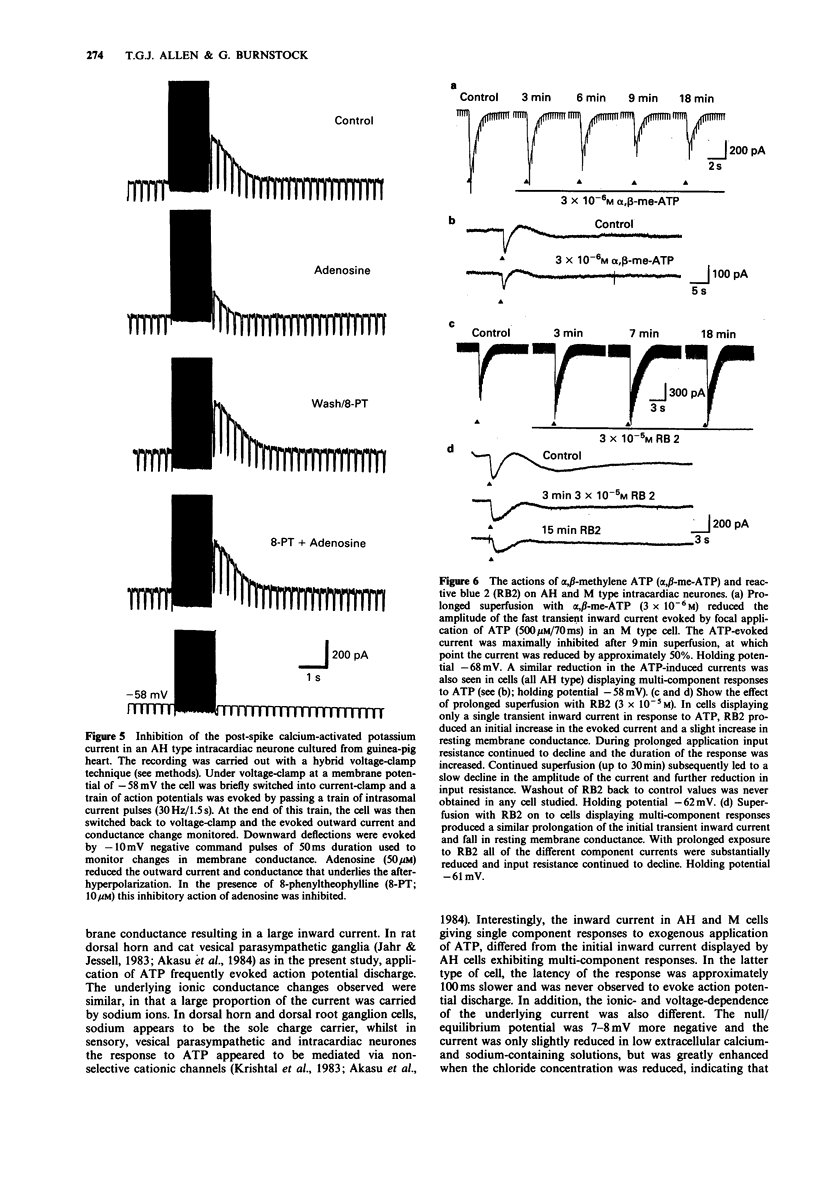
1. The actions of adenosine 5'-triphosphate (ATP) and related nucleotides and nucleosides on the membrane ion conductances of M and AH type intracardiac neurones cultured from ganglia within the atria and interatrial septum of newborn guinea-pig heart were studied with intracellular current- and voltage-clamp techniques. 2. Approximately 74% (120 out of 161) of AH type cells and 41% (5 out of 12) M cells responded to direct application of ATP (500 microM) onto their soma. 3. In 41% of M and 43% of AH type cells, focal application of ATP (500 microM) evoked rapid depolarization with an increase in conductance which frequently elicited action potential discharge. The underlying inward current had a null potential of -11.2 mV and was reduced in solutions containing low extracellular sodium and calcium but unaffected by reduced chloride-containing solutions. 4. In a further 31% of AH type cells, ATP evoked a multi-component response consisting of an initial depolarization followed by a hyperpolarization and a slow prolonged depolarization. The current underlying the initial depolarization resulted from an increase in conductance and had a null potential of -19.1 mV. The current was increased in low chloride-containing solutions and was only slightly reduced in low sodium- and calcium-containing solutions. The subsequent hyperpolarization and outward current resulted from an increase in membrane conductance and had a null potential of -88.5 mV, which was close to the potassium equilibrium potential in these cells. The slow depolarization and inward current was not associated with change in membrane conductance. 5. In less than 2% of AH cells, ATP evoked a second type of slow depolarization.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Hirai K., Koketsu K. Modulatory actions of ATP on membrane potentials of bullfrog sympathetic ganglion cells. Brain Res. 1983 Jan 10;258(2):313–317. doi: 10.1016/0006-8993(83)91157-5. [DOI] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Effect of adenosine triphosphate on the sensitivity of the nicotinic acetylcholine-receptor in the bullfrog sympathetic ganglion cell. Br J Pharmacol. 1985 Feb;84(2):525–531. doi: 10.1111/j.1476-5381.1985.tb12937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Shinnick-Gallagher P., Gallagher J. P. Adenosine mediates a slow hyperpolarizing synaptic potential in autonomic neurones. Nature. 1984 Sep 6;311(5981):62–65. doi: 10.1038/311062a0. [DOI] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987 Jul;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurones in culture. J Physiol. 1990 Mar;422:463–480. doi: 10.1113/jphysiol.1990.sp017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe R., Burnstock G. Fluorescent histochemical localisation of quinacrine-positive neurones in the guinea-pig and rabbit atrium. Cardiovasc Res. 1982 Jul;16(7):384–390. doi: 10.1093/cvr/16.7.384. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Greene R. W. Adenosine enhances afterhyperpolarization and accommodation in hippocampal pyramidal cells. Pflugers Arch. 1984 Nov;402(3):244–247. doi: 10.1007/BF00585506. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986 Jan 29;364(1):102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Hopwood A. M., Burnstock G. ATP mediates coronary vasoconstriction via P2x-purinoceptors and coronary vasodilatation via P2y-purinoceptors in the isolated perfused rat heart. Eur J Pharmacol. 1987 Apr 7;136(1):49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Hume R. I., Thomas S. A. Multiple actions of adenosine 5'-triphosphate on chick skeletal muscle. J Physiol. 1988 Dec;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVIN J. L., IRVIN E. M. The interaction of quinacrine with adenine nucleotides. J Biol Chem. 1954 Sep;210(1):45–56. [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983 Aug 25;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- KING T. S., COAKLEY J. B. The intrinsic nerve cells of the cardiac atria of mammals and man. J Anat. 1958 Jul;92(3):353–376. [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Morita K. Adenosine 5'-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol. 1989 Jan;408:373–390. doi: 10.1113/jphysiol.1989.sp017464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Hassall C. J., Burnstock G. Culture of intramural cardiac ganglia of the newborn guinea-pig. I. Neuronal elements. Cell Tissue Res. 1986;244(3):595–604. doi: 10.1007/BF00212539. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini S., Hoyle C. H., Burnstock G. An electrophysiological analysis of the effect of reactive blue 2, a putative P2-purinoceptor antagonist, on inhibitory junction potentials of rat caecum. Eur J Pharmacol. 1986 Aug 15;127(3):197–204. doi: 10.1016/0014-2999(86)90364-x. [DOI] [PubMed] [Google Scholar]
- Morita K., Katayama Y., Koketsu K., Akasu T. Actions of ATP on the soma of bullfrog primary afferent neurons and its modulating action on the GABA-induced response. Brain Res. 1984 Feb 20;293(2):360–363. doi: 10.1016/0006-8993(84)91243-5. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Purinergic inhibition in the small intestinal myenteric plexus of the guinea-pig. J Physiol. 1987 Jun;387:357–369. doi: 10.1113/jphysiol.1987.sp016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Excitation of single sensory neurones in the rat caudal trigeminal nucleus by iontophoretically applied adenosine 5'-triphosphate. Neurosci Lett. 1983 Jan 31;35(1):53–57. doi: 10.1016/0304-3940(83)90526-8. [DOI] [PubMed] [Google Scholar]
- Segal M. Intracellular analysis of a postsynaptic action of adenosine in the rat hippocampus. Eur J Pharmacol. 1982 Apr 23;79(3-4):193–199. doi: 10.1016/0014-2999(82)90625-2. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic neurotransmission and neuromodulation. Annu Rev Pharmacol Toxicol. 1983;23:397–411. doi: 10.1146/annurev.pa.23.040183.002145. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G. A., Belardinelli L. Sinus slowing and pacemaker shift caused by adenosine in rabbit SA node. Pflugers Arch. 1985 Jan;403(1):66–74. doi: 10.1007/BF00583284. [DOI] [PubMed] [Google Scholar]
- Yatani A., Goto M., Tsuda Y. Nature of catecholamine-like actions of ATP and other energy rich nucleotides on the bullfrog atrial muscle. Jpn J Physiol. 1978;28(1):47–61. doi: 10.2170/jjphysiol.28.47. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


