Abstract
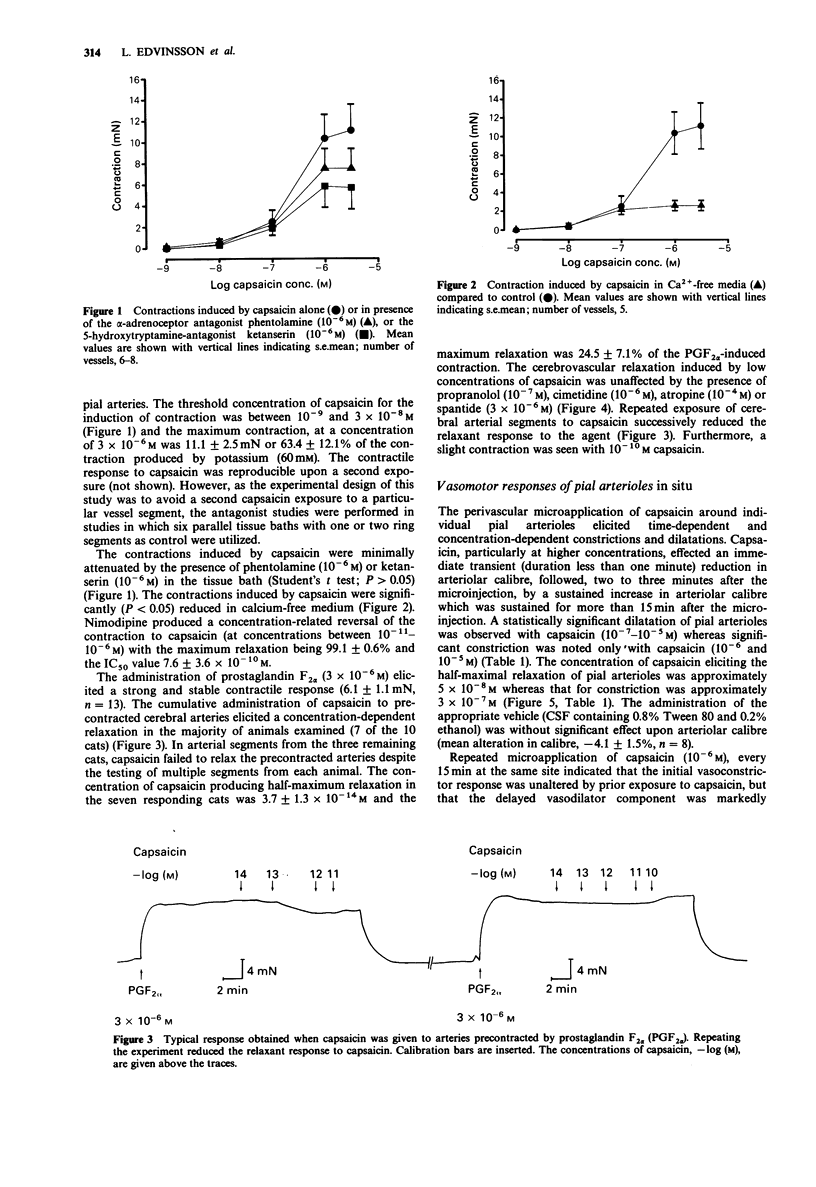
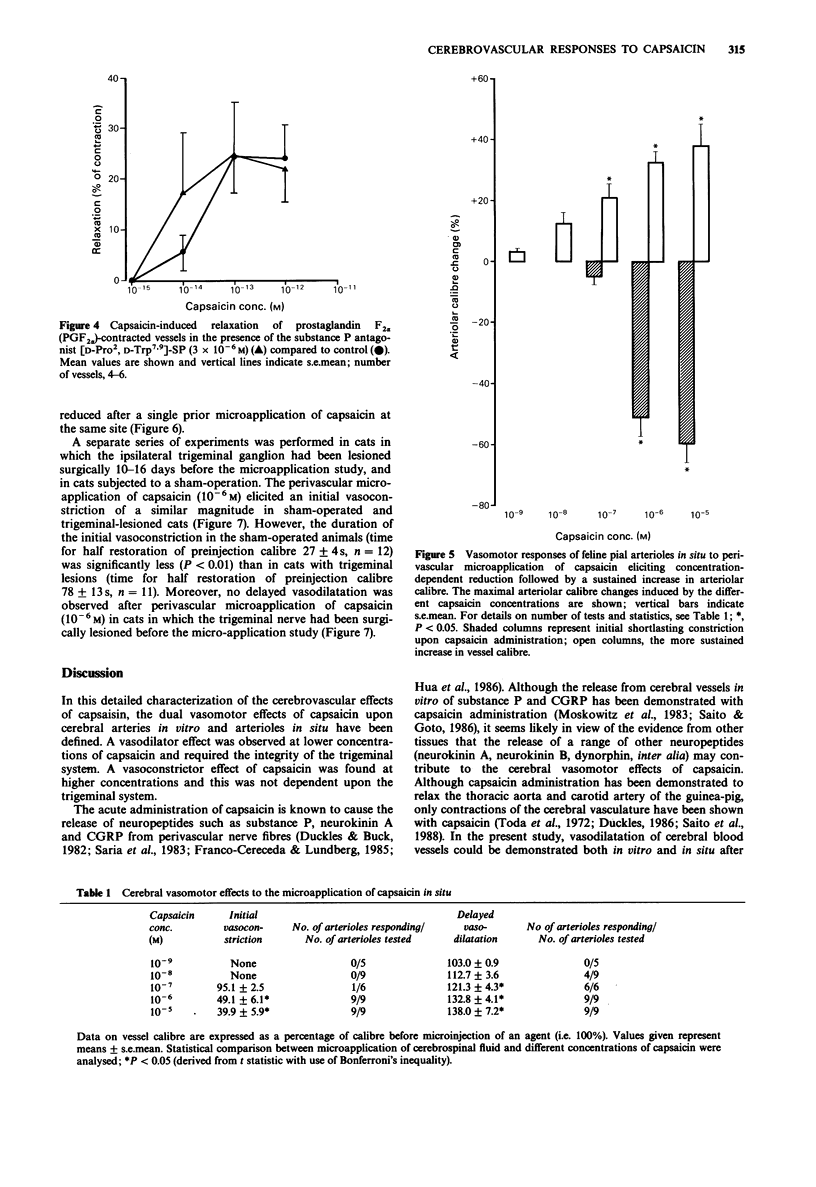
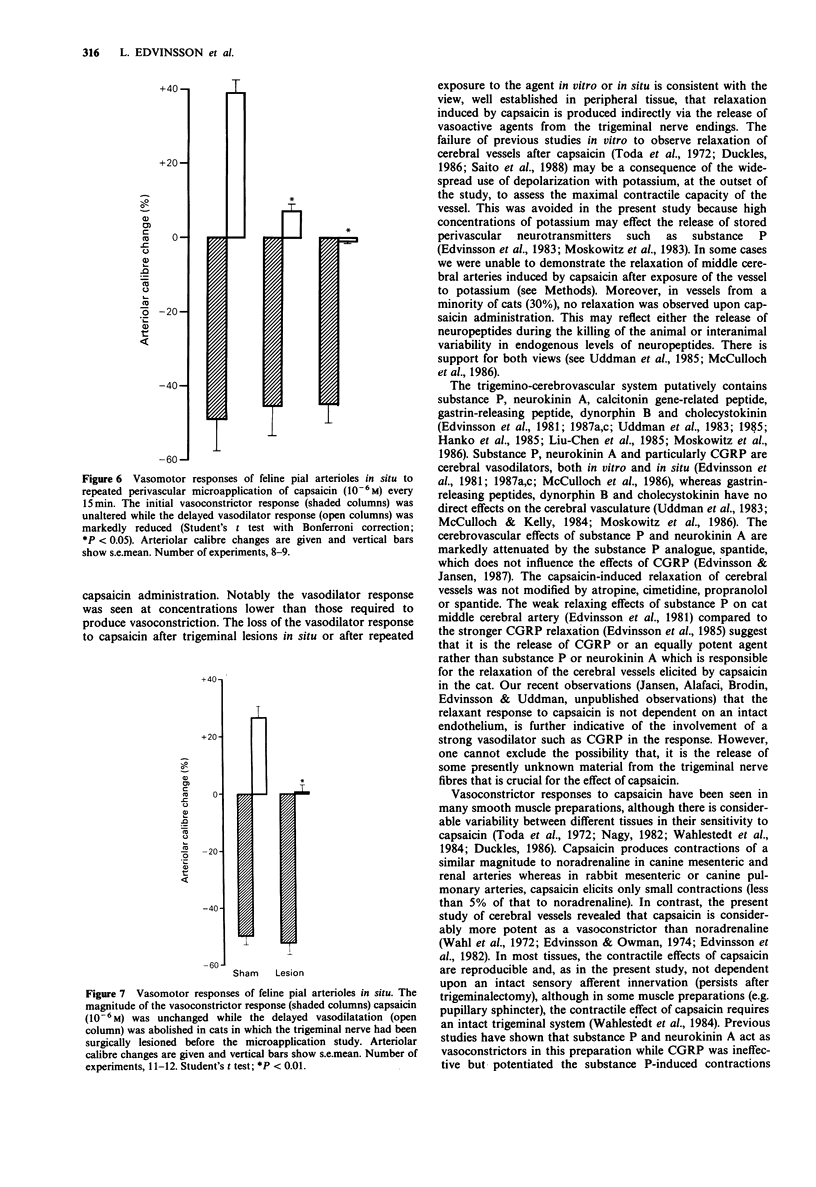
1. The cerebrovascular effects of capsaicin have been examined in vitro, in feline isolated cerebral arteries (circular segments, 2-3 mm long, 300-400 microns extended diameter) and, in situ, in pial arterioles (diameter 40-200 microns) on the cortical surface of chloralose-anaesthetized cats. 2. In isolated middle cerebral arteries, low concentrations of capsaicin (10(-14)-10(-10) M) effected a concentration-dependent relaxation of vessels precontracted with prostaglandin F2 alpha. This relaxant response was markedly attenuated by repeated administration of capsaicin but was minimally affected by the presence of atropine, propranolol, cimetidine or spantide in the tissue bath. 3. In isolated middle cerebral arteries, higher concentrations of capsaicin effected a marked concentration-dependent contraction. This contraction was not modified by 10(-6) M phentolamine or 10(-6) M ketanserin. A markedly reduced contraction by capsaicin was found upon the removal of calcium ions from the buffer solution. Also the calcium entry blocker nimodipine reversed the capsaicin-induced contraction. 4. Subarachnoid perivascular microapplication of capsaicin around individual pial arterioles in situ elicited a biphasic response (an immediate vasoconstriction followed by a sustained vasodilatation). The maximum vasoconstriction was a 60 +/- 6% reduction in diameter from base line and the maximum vasodilatation a 38 +/- 7% increase in diameter. Vasodilatation occurred at lower concentrations of capsaicin (EC50, approximately 5 x 10(-8) M) than those required for vasoconstriction (EC50 3 x 10(-7) M). 5. Trigeminal ganglionectomy 10-16 days before the microapplication abolished the in situ vasodilator effects of capsaicin (10(-6) M) applied perivascularly, but was without effect on the vasoconstrictor actions of this agent.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez S. Recording of microvascular dimensions with an image-splitter television microscope. J Appl Physiol. 1966 Jan;21(1):299–301. doi: 10.1152/jappl.1966.21.1.299. [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Buck S. H. Substance P in the cerebral vasculature: depletion by capsaicin suggests a sensory role. Brain Res. 1982 Aug 5;245(1):171–174. doi: 10.1016/0006-8993(82)90355-9. [DOI] [PubMed] [Google Scholar]
- Duckles S. P. Effects of capsaicin on vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1986 May;333(1):59–64. doi: 10.1007/BF00569661. [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Levitt B. Specificity of capsaicin treatment in the cerebral vasculature. Brain Res. 1984 Aug 6;308(1):141–144. doi: 10.1016/0006-8993(84)90925-9. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Ekman R., Jansen I., McCulloch J., Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987 Dec;7(6):720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Ekman R., Jansen I., Ottosson A., Uddman R. Peptide-containing nerve fibers in human cerebral arteries: immunocytochemistry, radioimmunoassay, and in vitro pharmacology. Ann Neurol. 1987 May;21(5):431–437. doi: 10.1002/ana.410210503. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B., Hamel E., Jansen I., Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985 Jul 31;58(2):213–217. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Jansen I. Characterization of tachykinin receptors in isolated basilar arteries of guinea-pig. Br J Pharmacol. 1987 Mar;90(3):553–559. doi: 10.1111/j.1476-5381.1987.tb11205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Feline cerebral veins and arteries: comparison of autonomic innervation and vasomotor responses. J Physiol. 1982 Apr;325:161–173. doi: 10.1113/jphysiol.1982.sp014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Owman C. Pharmacological characterization of adrenergic alpha and beta receptors mediating the vasomotor responses of cerebral arteries in vitro. Circ Res. 1974 Dec;35(6):835–849. doi: 10.1161/01.res.35.6.835. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Rosendal-Helgesen S., Uddman R. Substance P: localization, concentration and release in cerebral arteries, choroid plexus and dura mater. Cell Tissue Res. 1983;234(1):1–7. doi: 10.1007/BF00217397. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M. Calcitonin gene-related peptide (CGRP) and capsaicin-induced stimulation of heart contractile rate and force. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):146–151. doi: 10.1007/BF00634231. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Hanko J., Hardebo J. E., Kåhrström J., Owman C., Sundler F. Calcitonin gene-related peptide is present in mammalian cerebrovascular nerve fibres and dilates pial and peripheral arteries. Neurosci Lett. 1985 Jun 4;57(1):91–95. doi: 10.1016/0304-3940(85)90045-x. [DOI] [PubMed] [Google Scholar]
- Harper A. M., MacKenzie E. T. Effects of 5-hydroxytryptamine on pial arteriolar calibre in anaesthetized cats. J Physiol. 1977 Oct;271(3):735–746. doi: 10.1113/jphysiol.1977.sp012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X. Y., Saria A., Gamse R., Theodorsson-Norheim E., Brodin E., Lundberg J. M. Capsaicin induced release of multiple tachykinins (substance P, neurokinin A and eledoisin-like material) from guinea-pig spinal cord and ureter. Neuroscience. 1986 Sep;19(1):313–319. doi: 10.1016/0306-4522(86)90024-2. [DOI] [PubMed] [Google Scholar]
- Hua X. Y., Theodorsson-Norheim E., Brodin E., Lundberg J. M., Hökfelt T. Multiple tachykinins (neurokinin A, neuropeptide K and substance P) in capsaicin-sensitive sensory neurons in the guinea-pig. Regul Pept. 1985 Dec;13(1):1–19. doi: 10.1016/0167-0115(85)90082-5. [DOI] [PubMed] [Google Scholar]
- Högestätt E. D., Andersson K. E., Edvinsson L. Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small blood vessels. Acta Physiol Scand. 1983 Jan;117(1):49–61. doi: 10.1111/j.1748-1716.1983.tb07178.x. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Han D. H., Moskowitz M. A. Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience. 1983 Aug;9(4):803–808. doi: 10.1016/0306-4522(83)90268-3. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Norregaard T. V., Moskowitz M. A. Some cholecystokinin-8 immunoreactive fibers in large pial arteries originate from trigeminal ganglion. Brain Res. 1985 Dec 16;359(1-2):166–176. doi: 10.1016/0006-8993(85)91425-8. [DOI] [PubMed] [Google Scholar]
- Mayberg M. R., Zervas N. T., Moskowitz M. A. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984 Feb 10;223(1):46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Kelly P. A. Effects of cholecystokinin octapeptide on pial arteriolar diameter. J Cereb Blood Flow Metab. 1984 Dec;4(4):625–628. doi: 10.1038/jcbfm.1984.88. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Uddman R., Kingman T. A., Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. A., Brezina L. R., Kuo C. Dynorphin B-containing perivascular axons and sensory neurotransmitter mechanisms in brain blood vessels. Cephalalgia. 1986 Jun;6(2):81–86. doi: 10.1046/j.1468-2982.1986.0602081.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Reid J., McCulloch J. Capsaicin and blood-brain barrier permeability. Neurosci Lett. 1987 Oct 16;81(1-2):165–170. doi: 10.1016/0304-3940(87)90359-4. [DOI] [PubMed] [Google Scholar]
- Saito A., Goto K. Depletion of calcitonin gene-related peptide (CGRP) by capsaicin in cerebral arteries. J Pharmacobiodyn. 1986 Jul;9(7):613–619. doi: 10.1248/bpb1978.9.613. [DOI] [PubMed] [Google Scholar]
- Saito A., Masaki T., Lee T. J., Goto K. Effects of capsaicin on the contractility and peptide-containing nerves of large cerebral arteries of the cat. Arch Int Pharmacodyn Ther. 1988 Sep-Oct;295:194–203. [PubMed] [Google Scholar]
- Saria A., Lundberg J. M., Hua X., Lembeck F. Capsaicin-induced substance P release and sensory control of vascular permeability in the guinea-pig ureter. Neurosci Lett. 1983 Oct 31;41(1-2):167–172. doi: 10.1016/0304-3940(83)90241-0. [DOI] [PubMed] [Google Scholar]
- Toda N., Usui H., Nishino N., Fujiwara M. Cardiovascular effects of capsaicin in dogs and rabbits. J Pharmacol Exp Ther. 1972 Jun;181(3):512–521. [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekman R., Kingman T., McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985 Nov 20;62(1):131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Owman C., Sundler F. Nerve fibres containing gastrin-releasing peptide around pial vessels. J Cereb Blood Flow Metab. 1983 Sep;3(3):386–390. doi: 10.1038/jcbfm.1983.56. [DOI] [PubMed] [Google Scholar]
- Wahl M., Kuschinsky W., Bosse O., Olesen J., Lassen N. A., Ingvar D. H., Michaelis J., Thurau K. Effect of 1-norepinephrine on the diameter of pial arterioles and arteries in the cat. Circ Res. 1972 Aug;31(2):248–256. doi: 10.1161/01.res.31.2.248. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Beding B., Ekman R., Oksala O., Stjernschantz J., Håkanson R. Calcitonin gene-related peptide in the eye: release by sensory nerve stimulation and effects associated with neurogenic inflammation. Regul Pept. 1986 Dec 22;16(2):107–115. doi: 10.1016/0167-0115(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Bynke G., Håkanson R. Pupillary constriction by bradykinin and capsaicin: mode of action. Eur J Pharmacol. 1984 Nov 27;106(3):577–583. doi: 10.1016/0014-2999(84)90061-x. [DOI] [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S., Mulderry P. K., Ghatei M. A., McGregor G. P., Bloom S. R., Polak J. M. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986 Aug;16(4):289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]


