Abstract
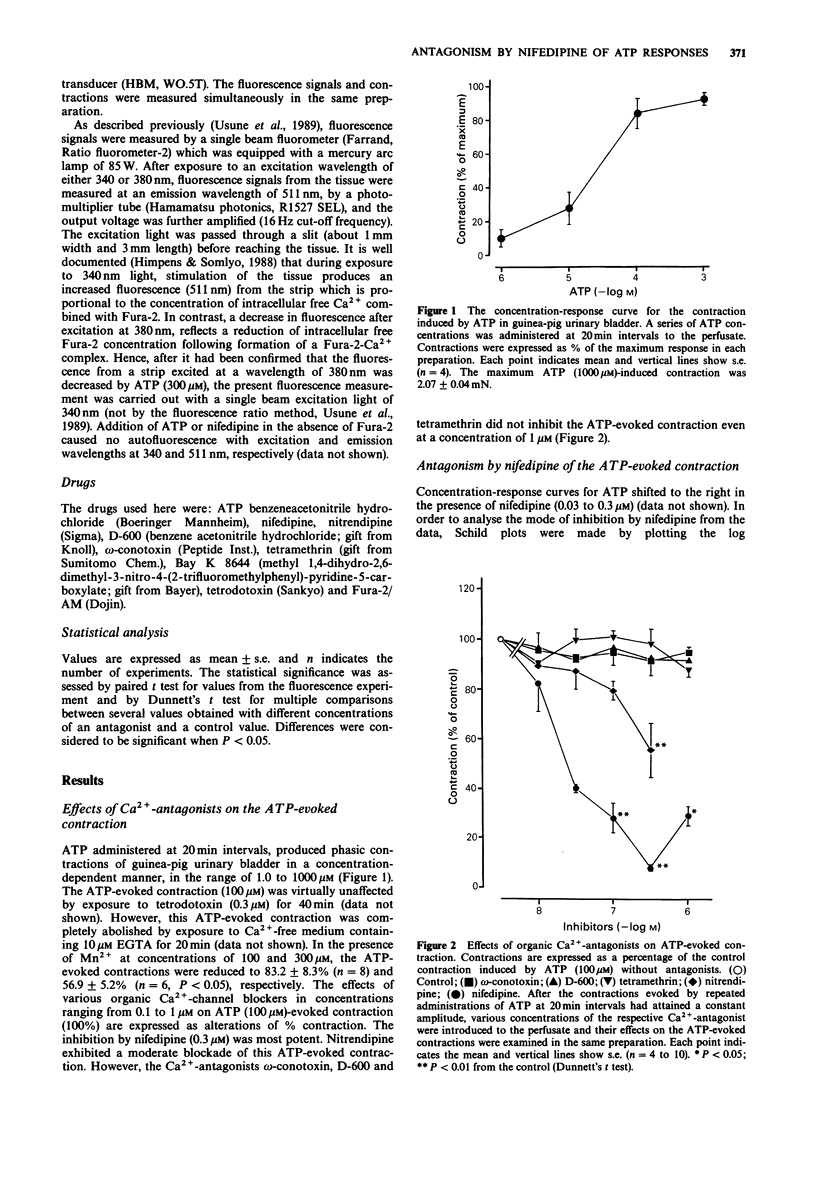
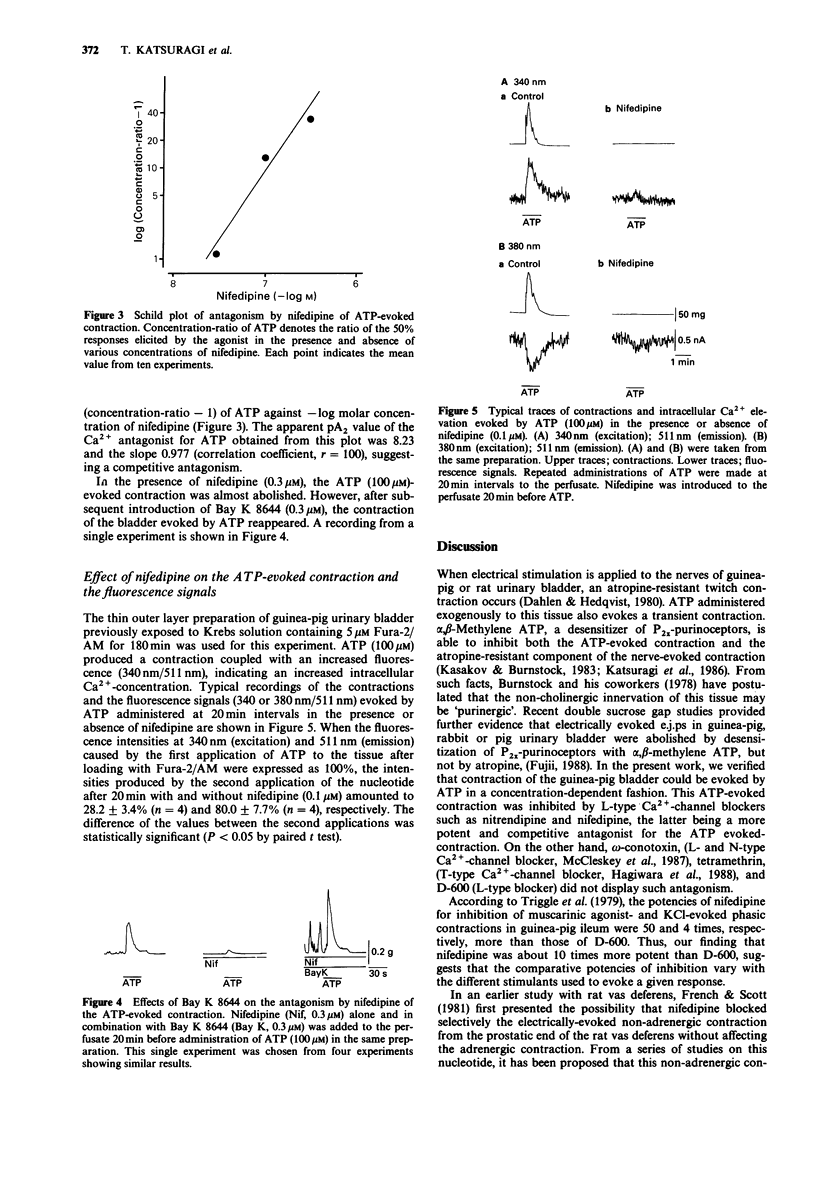
1. The effects of Ca2(+)-antagonists, especially nifedipine, on contraction and increase of intracellular Ca2+ (Fura-2/AM method) evoked by ATP were evaluated in a thin outer layer segment of guinea-pig urinary bladder. 2. The ATP-evoked contraction was markedly inhibited by dihydropyridine-type Ca2(+)-antagonists, such as nifedipine and nitrendipine, but not by D-600, omega-conotoxin and tetramethrin. 3. This antagonism by nifedipine of ATP-evoked contractions was competitive from the Schild plot analysis, the pA2 value being 8.23. The reduction of ATP-evoked contraction by nifedipine (0.1 microM) was fully reversed by administration of Bay K 8644 (0.1 microM). 4. ATP (100 microM) caused an increase of fluorescence brightness after loading Fura-2/AM, which was coupled with a contraction of the bladder. Both the contraction and the elevation of intracellular Ca2+ evoked evoked by the nucleotide were completely antagonized by nifedipine. by the nucleotide were completely antagonized by nifedipine. 5. These results suggest that ATP may activate the dihydropyridine-sensitive, voltage-dependent Ca2(+)-channels in a direct or indirect fashion and, thereby, elicit a contraction of the bladder.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Blakeley A. G., Brown D. A., Cunnane T. C., French A. M., McGrath J. C., Scott N. C. Effects of nifedipine on electrical and mechanical responses of rat and guinea pig vas deferens. Nature. 1981 Dec 24;294(5843):759–761. doi: 10.1038/294759a0. [DOI] [PubMed] [Google Scholar]
- Bulloch J. M., McGrath J. C. Selective blockade by nifedipine of 'purinergic' rather than adrenergic nerve-mediated vasopressor responses in the pithed rat. Br J Pharmacol. 1988 Nov;95(3):695–700. doi: 10.1111/j.1476-5381.1988.tb11695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurones. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. The effect of alpha, beta-methylene ATP on the depolarization evoked by noradrenaline (gamma-adrenoceptor response) and ATP in the immature rat basilar artery. Br J Pharmacol. 1986 May;88(1):6–8. doi: 10.1111/j.1476-5381.1986.tb09464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén S. E., Hedqvist P. ATP, beta-gamma-methylene-ATP, and adenosine inhibit non-cholinergic non-adrenergic transmission in rat urinary bladder. Acta Physiol Scand. 1980 Jun;109(2):137–142. doi: 10.1111/j.1748-1716.1980.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Danziger R. S., Raffaeli S., Moreno-Sanchez R., Sakai M., Capogrossi M. C., Spurgeon H. A., Hansford R. G., Lakatta E. G. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988 Aug;9(4):193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. M., Scott N. C. A comparison of the effects of nifedipine and verapamil on rat vas deferens. Br J Pharmacol. 1981 Jun;73(2):321–323. doi: 10.1111/j.1476-5381.1981.tb10424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Morita K. Adenosine 5'-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol. 1989 Jan;408:373–390. doi: 10.1113/jphysiol.1989.sp017464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi T., Kuratomi L., Furukawa T. Clonidine-evoked selective P1-purinoceptor antagonism of contraction of guinea-pig urinary bladder. Eur J Pharmacol. 1986 Feb 11;121(1):119–122. doi: 10.1016/0014-2999(86)90400-0. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Tokunaga T., Miyamoto K., Kuratomi L., Furukawa T. Norepinephrine and adenosine triphosphate release in different ratio from guinea pig vas deferens by high potassium chloride, ouabain and monensin. J Pharmacol Exp Ther. 1988 Oct;247(1):302–308. [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H., Stajärne L. Evidence that most noradrenaline is stored without ATP in sympathetic large dense core nerve vesicles. Nature. 1974 Jun 28;249(460):843–845. doi: 10.1038/249843a0. [DOI] [PubMed] [Google Scholar]
- Lew M. J., White T. D. Release of endogenous ATP during sympathetic nerve stimulation. Br J Pharmacol. 1987 Oct;92(2):349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara H., Suzuki H. Pre- and post-junctional effects of adenosine triphosphate on noradrenergic transmission in the rabbit ear artery. J Physiol. 1987 Aug;389:423–440. doi: 10.1113/jphysiol.1987.sp016664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. ATP regulation of the slow calcium channels in vascular smooth muscle cells of guinea pig mesenteric artery. Circ Res. 1989 Jan;64(1):145–154. doi: 10.1161/01.res.64.1.145. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Discrete events measure single quanta of adenosine 5'-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1984 Sep;13(1):21–28. doi: 10.1016/0306-4522(84)90256-2. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Differential blockade of ATP, noradrenaline and electrically evoked contractions of the rat vas deferens by nifedipine. Eur J Pharmacol. 1981 Sep 24;74(4):373–376. doi: 10.1016/0014-2999(81)90058-3. [DOI] [PubMed] [Google Scholar]
- Triggle C. R., Swamy V. C., Triggle D. J. Calcium antagonists and contractile responses in rat vas deferens and guinea pig ileal smooth muscle. Can J Physiol Pharmacol. 1979 Aug;57(8):804–818. doi: 10.1139/y79-124. [DOI] [PubMed] [Google Scholar]
- Usune S., Katsuragi T., Furukawa T. Two phases of the prostaglandin F2 alpha-induced contraction in guinea-pig taenia coli involve different Ca2+ channels. Naunyn Schmiedebergs Arch Pharmacol. 1989 Oct;340(4):437–441. doi: 10.1007/BF00167046. [DOI] [PubMed] [Google Scholar]
- Usune S., Katsuragi T., Sakamoto Y., Furukawa T. Contribution of Na+ and membrane depolarization to contraction induced by adrenaline in the guinea pig vas deferens. Can J Physiol Pharmacol. 1986 Jun;64(6):720–723. doi: 10.1139/y86-121. [DOI] [PubMed] [Google Scholar]
- Wakui M., Inomata H. Evidence for an increase in membrane conductance during adenosine triphosphate-induced depolarization in the guinea-pig vas deferens. Pflugers Arch. 1985 Jan;403(1):112–114. doi: 10.1007/BF00583291. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., Fedan J. S., Colby J., Hogaboom G. K., O'Donnell J. P. Evidence for a contribution by purines to the neurogenic response of the guinea-pig urinary bladder. Eur J Pharmacol. 1983 Mar 4;87(4):415–422. doi: 10.1016/0014-2999(83)90080-8. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


