Abstract
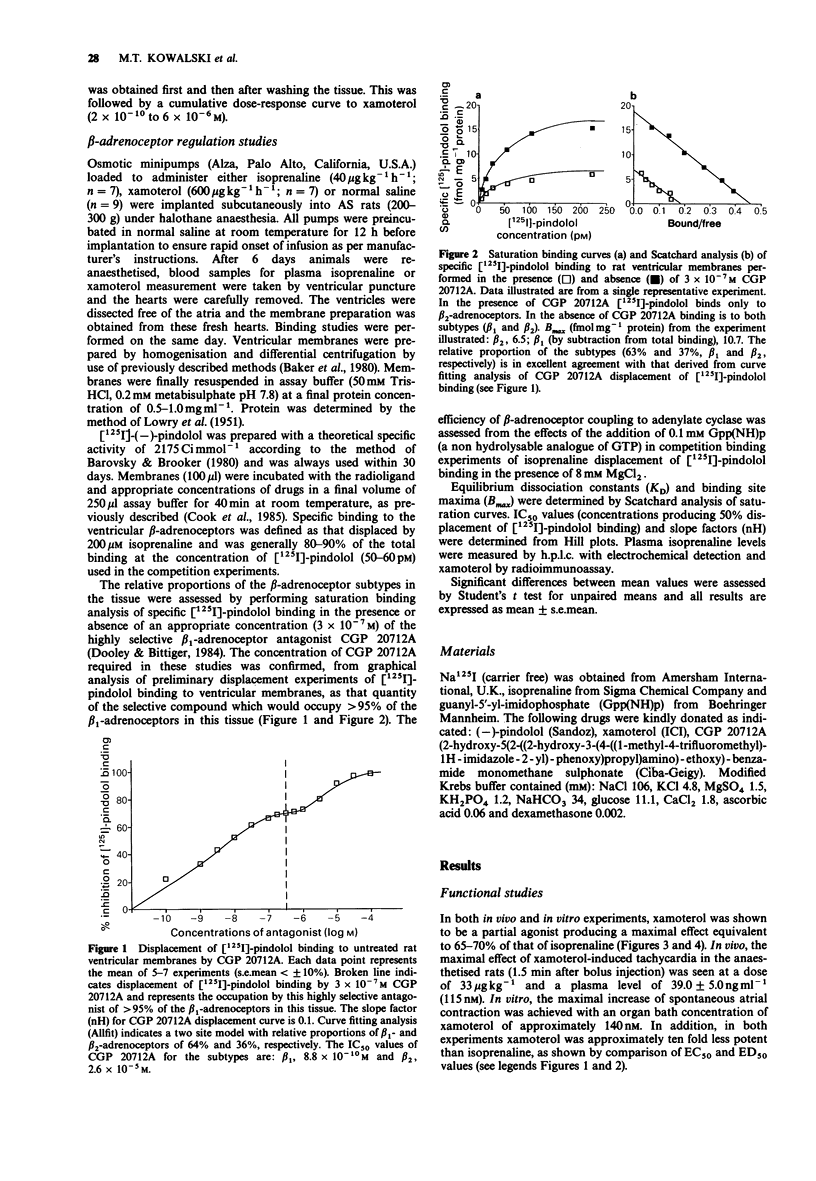
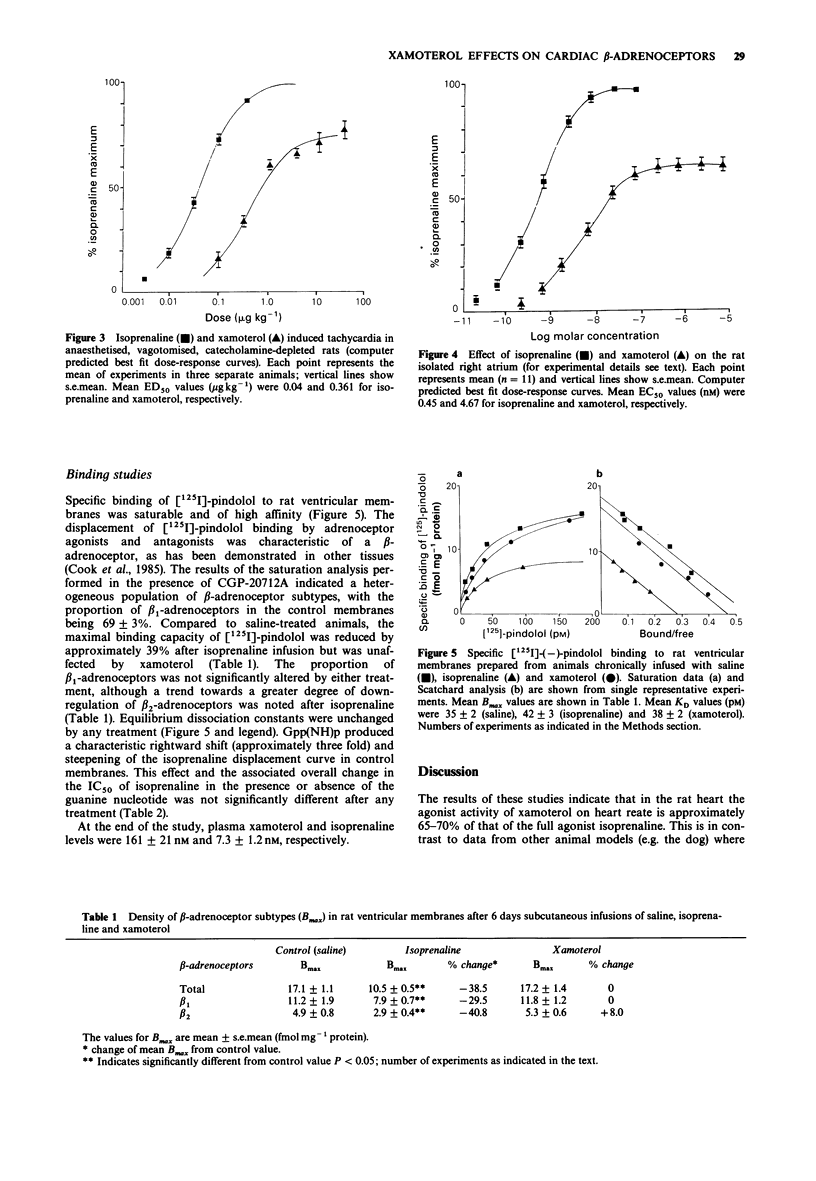
1. The effects of the beta 1-selective partial agonist xamoterol and the full agonist isoprenaline on rat cardiac beta-adrenoceptors were compared in functional studies of heart rate response in vivo and in vitro. In addition, the ability of both agents to cause receptor down-regulation in the rat heart following chronic (6 days) subcutaneous infusions was assessed by radioligand binding with [125I]-pindolol. 2. In the functional studies, xamoterol produced a maximal effect equivalent to approximately 65% of that of isoprenaline and was overall less potent than the full agonist. 3. Compared to saline control, the density of beta-adrenoceptors was reduced approximately 39% in ventricular membranes prepared from animals after 6 days of isoprenaline infusion but was unaffected by xamoterol. The relative proportions of the beta-adrenoceptor subtypes were unchanged by either active treatment. 4. Plasma xamoterol level at the end of the infusion period was equivalent to that associated with maximum tachycardia in vivo and to the concentration producing maximal stimulation of the rat isolated atrium in vitro. Thus suggesting 100% beta-adrenoceptor occupancy during the period of xamoterol infusion. 5. These results indicate that in this animal model xamoterol does not induce cardiac beta-adrenoceptor down-regulation during chronic treatment, with doses that produce a maximal functional response both in vitro and in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarons R. D., Molinoff P. B. Changes in the density of beta adrenergic receptors in rat lymphocytes, heart and lung after chronic treatment with propranolol. J Pharmacol Exp Ther. 1982 May;221(2):439–443. [PubMed] [Google Scholar]
- Baker S. P., Boyd H. M., Potter L. T. Distribution and function of beta-adrenoceptors in different chambers of the canine heart. Br J Pharmacol. 1980 Jan;68(1):57–63. doi: 10.1111/j.1476-5381.1980.tb10698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barovsky K., Brooker G. (-)-[125I]-iodopindolol, a new highly selective radioiodinated beta-adrenergic receptor antagonist: measurement of beta-receptors on intact rat astrocytoma cells. J Cyclic Nucleotide Res. 1980;6(4):297–307. [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bristow M. R. Myocardial beta-adrenergic receptor downregulation in heart failure. Int J Cardiol. 1984 May;5(5):648–652. doi: 10.1016/0167-5273(84)90179-7. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Alexander R. W., Williams G. H., Rude R. E., Holman B. L., Konstam M. A., Wynne J., Mudge G. H., Jr, Braunwald E. Decreased lymphocyte beta-adrenergic-receptor density in patients with heart failure and tolerance to the beta-adrenergic agonist pirbuterol. N Engl J Med. 1981 Jul 23;305(4):185–190. doi: 10.1056/NEJM198107233050402. [DOI] [PubMed] [Google Scholar]
- Cook N., Nahorski S. R., Barnett D. B. (-)-[125I]pindolol binding to the human platelet beta-adrenoceptor: characterisation and agonist interactions. Eur J Pharmacol. 1985 Jul 17;113(2):247–254. doi: 10.1016/0014-2999(85)90742-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Shiina A., Toyo-Oka T., Hosoda S., Kondo K. The cardiovascular effects of xamoterol, a beta 1-adrenoceptor partial agonist, in healthy volunteers at rest. Br J Clin Pharmacol. 1986 Mar;21(3):259–265. doi: 10.1111/j.1365-2125.1986.tb05188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minneman K. P., Pittman R. N., Molinoff P. B. Beta-adrenergic receptor subtypes: properties, distribution, and regulation. Annu Rev Neurosci. 1981;4:419–461. doi: 10.1146/annurev.ne.04.030181.002223. [DOI] [PubMed] [Google Scholar]
- Nerme V., Severne Y., Abrahamsson T., Vauquelin G. Endogenous noradrenaline masks beta-adrenergic receptors in rat heart membranes via tight agonist binding. Biochem Pharmacol. 1985 Aug 15;34(16):2917–2922. doi: 10.1016/0006-2952(85)90016-4. [DOI] [PubMed] [Google Scholar]
- Nuttall A., Snow H. M. The cardiovascular effects of ICI 118,587: A beta 1-adrenoceptor partial agonist. Br J Pharmacol. 1982 Oct;77(2):381–388. doi: 10.1111/j.1476-5381.1982.tb09309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


