Abstract
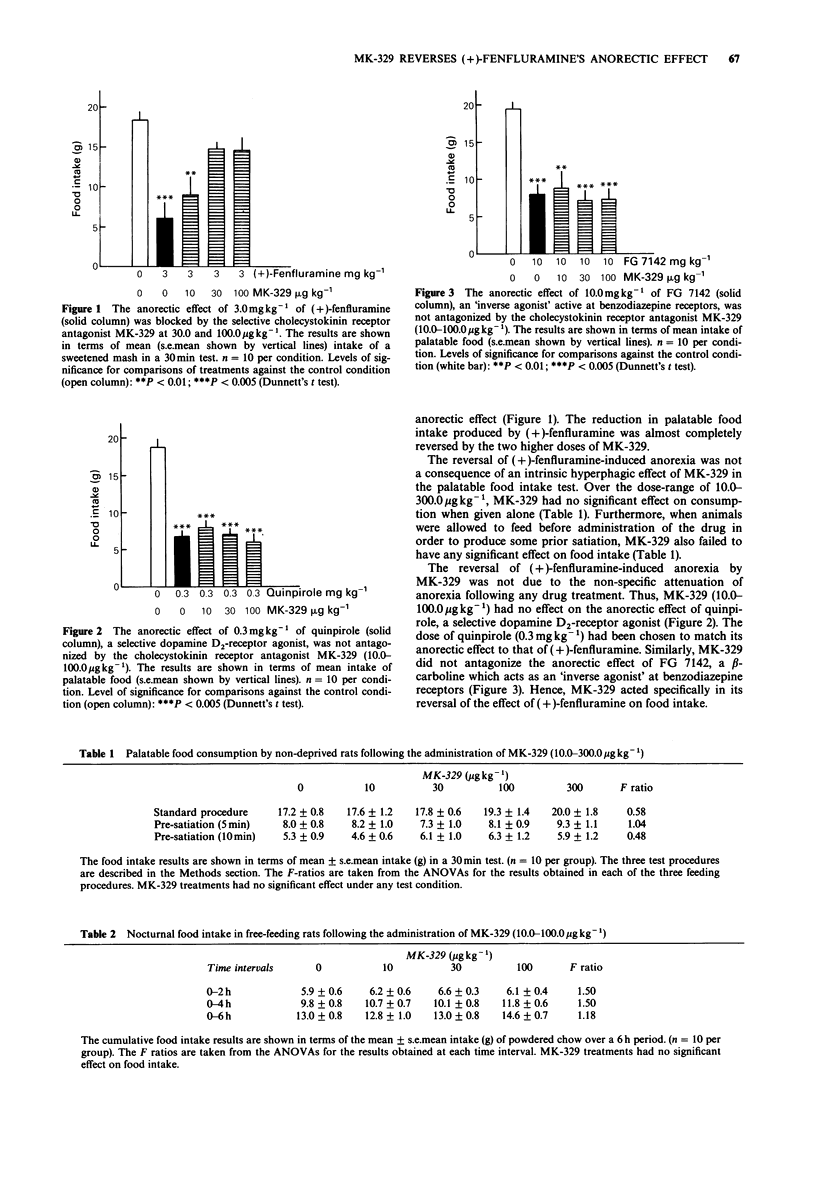
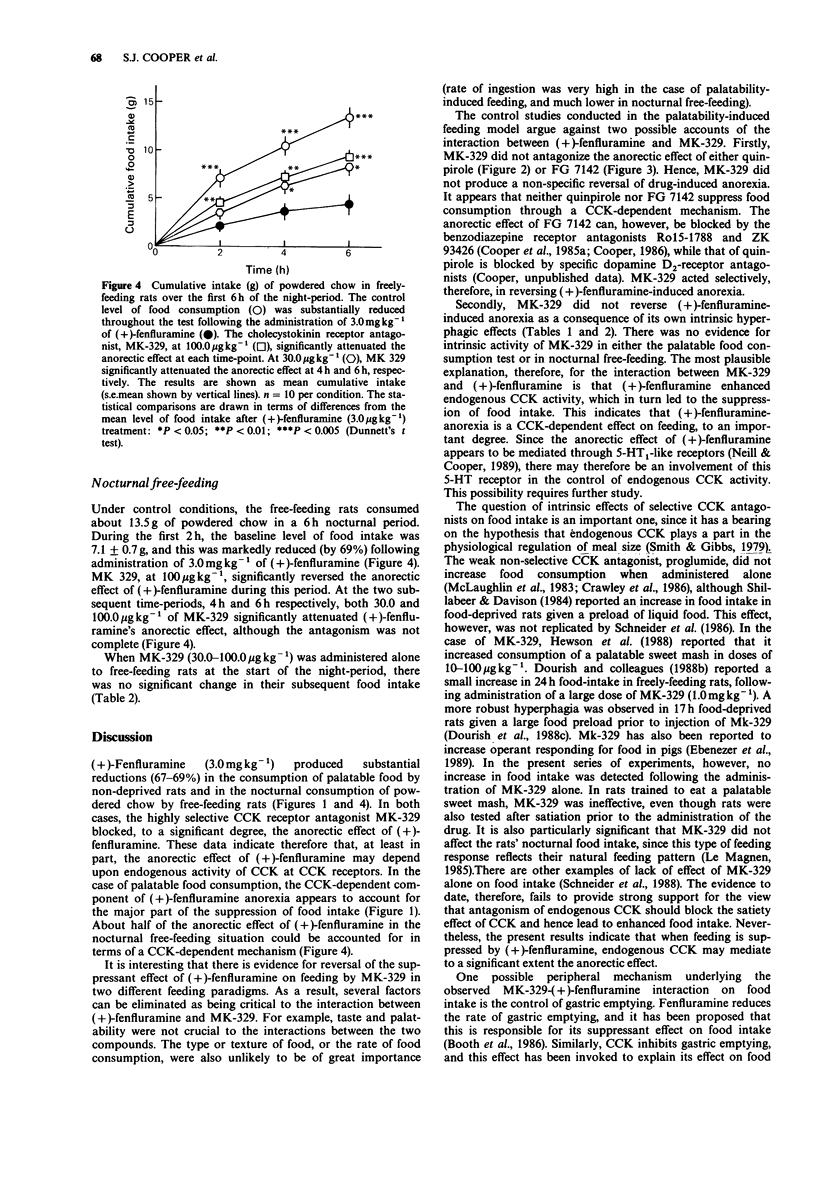
1. Experiments were conducted to determine whether or not the effect of (+)-fenfluramine (3.0 mg kg-1, i.p.) on food intake can be antagonized by the selective cholecystokinin receptor antagonist MK-239 (formerly L364,718; (3S(-)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1-H-1,4-benzodiazepin++ +-3-yl)-1H- indole-2-carboxamide). Two feeding paradigms were employed. In the first, non-deprived rats were familiarized with eating a highly palatable, sweetened mash in a 30 min test. In the second, freely-feeding rats were trained to consume powdered chow in their home-cages, and their intake was monitored over the first 6 h of the night-period. 2. In doses of 30.0 and 100.0 micrograms kg-1, s.c., MK-329 almost completely blocked the anorectic effect of (+)-fenfluramine in the palatable food intake test. These doses of MK-329 have previously been reported to antagonize the anorectic effect produced by exogenous cholecystokinin-octapeptide (CCK8) in rats. Both doses of MK-329 were also effective in significantly attenuating the anorectic effect of (+)-fenfluramine in nocturnal free-feeding animals over a 6 h-period. 3. MK-329 (10.0-100.0 micrograms kg-1, s.c.) failed to antagonize the anorectic effect of either the specific dopamine D2-receptor agonist quinpirole (0.3 mg kg-1, s.c.) or the beta-carboline FG 7142 (10.0 mg kg-1, i.p.) in the palatable food intake test.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baile C. A., McLaughlin C. L., Della-Fera M. A. Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev. 1986 Jan;66(1):172–234. doi: 10.1152/physrev.1986.66.1.172. [DOI] [PubMed] [Google Scholar]
- Baker B. J., Duggan J. P., Barber D. J., Booth D. A. Effects of dl-fenfluramine and xylamidine on gastric emptying of maintenance diet in freely feeding rats. Eur J Pharmacol. 1988 May 20;150(1-2):137–142. doi: 10.1016/0014-2999(88)90759-5. [DOI] [PubMed] [Google Scholar]
- Booth D. A., Gibson E. L., Baker B. J. Gastromotor mechanism of fenfluramine anorexia. Appetite. 1986;7 (Suppl):57–69. doi: 10.1016/s0195-6663(86)80052-6. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. J., Barber D. J., Gilbert D. B., Moores W. R. Benzodiazepine receptor ligands and the consumption of a highly palatable diet in non-deprived male rats. Psychopharmacology (Berl) 1985;86(3):348–355. doi: 10.1007/BF00432227. [DOI] [PubMed] [Google Scholar]
- Cooper S. J. Hyperphagic and anorectic effects of beta-carbolines in a palatable food consumption test: comparisons with triazolam and quazepam. Eur J Pharmacol. 1986 Jan 29;120(3):257–265. doi: 10.1016/0014-2999(86)90466-8. [DOI] [PubMed] [Google Scholar]
- Cooper S. J., Moores W. R., Jackson A., Barber D. J. Effects of tifluadom on food consumption compared with chlordiazepoxide and kappa agonists in the rat. Neuropharmacology. 1985 Sep;24(9):877–883. doi: 10.1016/0028-3908(85)90039-5. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Stivers J. A., Hommer D. W., Skirboll L. R., Paul S. M. Antagonists of central and peripheral behavioral actions of cholecystokinin octapeptide. J Pharmacol Exp Ther. 1986 Feb;236(2):320–330. [PubMed] [Google Scholar]
- Dourish C. T., Clark M. L., Fletcher A., Iversen S. D. Evidence that blockade of post-synaptic 5-HT1 receptors elicits feeding in satiated rats. Psychopharmacology (Berl) 1989;97(1):54–58. doi: 10.1007/BF00443413. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., Cooper S. J., Gilbert F., Coughlan J., Iversen S. D. The 5-HT1A agonist 8-OH-DPAT increases consumption of palatable wet mash and liquid diets in the rat. Psychopharmacology (Berl) 1988;94(1):58–63. doi: 10.1007/BF00735881. [DOI] [PubMed] [Google Scholar]
- Enzi G., Crepaldi G., Inelmen E. M., Bruni R., Baggio B. Efficacy and safety of dexfenfluramine in obese patients: a multicenter study. Clin Neuropharmacol. 1988;11 (Suppl 1):S173–S178. [PubMed] [Google Scholar]
- Evans B. E., Bock M. G., Rittle K. E., DiPardo R. M., Whitter W. L., Veber D. F., Anderson P. S., Freidinger R. M. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4918–4922. doi: 10.1073/pnas.83.13.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T., Dimaline R., Peikin S., Dockray G. J. Action of the cholecystokinin antagonist L364,718 on gastric emptying in the rat. Am J Physiol. 1988 Nov;255(5 Pt 1):G685–G689. doi: 10.1152/ajpgi.1988.255.5.G685. [DOI] [PubMed] [Google Scholar]
- Hewson G., Leighton G. E., Hill R. G., Hughes J. The cholecystokinin receptor antagonist L364,718 increases food intake in the rat by attenuation of the action of endogenous cholecystokinin. Br J Pharmacol. 1988 Jan;93(1):79–84. doi: 10.1111/j.1476-5381.1988.tb11407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson P. H., Donohoe T. P., Curzon G. Infusion of the 5-hydroxytryptamine agonists RU24969 and TFMPP into the paraventricular nucleus of the hypothalamus causes hypophagia. Psychopharmacology (Berl) 1988;95(4):550–552. doi: 10.1007/BF00172974. [DOI] [PubMed] [Google Scholar]
- Jackson A., Cooper S. J. Effects of kappa opiate agonists on palatable food consumption in non-deprived rats, with and without food preloads. Brain Res Bull. 1985 Oct;15(4):391–396. doi: 10.1016/0361-9230(85)90007-3. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96(1):93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Dourish C. T., Curzon G. 5-HT1B agonists induce anorexia at a postsynaptic site. Eur J Pharmacol. 1987 Sep 23;141(3):429–435. doi: 10.1016/0014-2999(87)90561-9. [DOI] [PubMed] [Google Scholar]
- Khosla S., Crawley J. N. Potency of L-364,718 as an antagonist of the behavioral effects of peripherally administered cholecystokinin. Life Sci. 1988;42(2):153–159. doi: 10.1016/0024-3205(88)90678-9. [DOI] [PubMed] [Google Scholar]
- Larue-Achagiotis C., Le Magnen J. Effect of long-term insulin on body weight and food intake: intravenous versus intraperitoneal routes. Appetite. 1985 Dec;6(4):319–329. doi: 10.1016/s0195-6663(85)80001-5. [DOI] [PubMed] [Google Scholar]
- Lotti V. J., Pendleton R. G., Gould R. J., Hanson H. M., Chang R. S., Clineschmidt B. V. In vivo pharmacology of L-364,718, a new potent nonpeptide peripheral cholecystokinin antagonist. J Pharmacol Exp Ther. 1987 Apr;241(1):103–109. [PubMed] [Google Scholar]
- McLaughlin C. L., Peikin S. R., Baile C. A. Feeding behavior response of Zucker rats to proglumide, a CCK receptor antagonist. Pharmacol Biochem Behav. 1983 Jun;18(6):879–883. doi: 10.1016/s0091-3057(83)80009-4. [DOI] [PubMed] [Google Scholar]
- Moran T. H., McHugh P. R. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol. 1982 May;242(5):R491–R497. doi: 10.1152/ajpregu.1982.242.5.R491. [DOI] [PubMed] [Google Scholar]
- Neill J. C., Cooper S. J. Evidence that d-fenfluramine anorexia is mediated by 5-HT1 receptors. Psychopharmacology (Berl) 1989;97(2):213–218. doi: 10.1007/BF00442252. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X., Kissileff H. R., Thornton J., Smith G. P. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol Behav. 1982 Oct;29(4):627–630. doi: 10.1016/0031-9384(82)90230-x. [DOI] [PubMed] [Google Scholar]
- Rowland N. E., Carlton J. Neurobiology of an anorectic drug: fenfluramine. Prog Neurobiol. 1986;27(1):13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Russell G. F., Checkley S. A., Feldman J., Eisler I. A controlled trial of d-fenfluramine in bulimia nervosa. Clin Neuropharmacol. 1988;11 (Suppl 1):S146–S159. [PubMed] [Google Scholar]
- Schneider L. H., Gibbs J., Smith G. P. Proglumide fails to increase food intake after an ingested preload. Peptides. 1986 Jan-Feb;7(1):135–140. doi: 10.1016/0196-9781(86)90073-2. [DOI] [PubMed] [Google Scholar]
- Shillabeer G., Davison J. S. The cholecystokinin antagonist, proglumide, increases food intake in the rat. Regul Pept. 1984 Apr;8(3):171–176. doi: 10.1016/0167-0115(84)90058-2. [DOI] [PubMed] [Google Scholar]
- Smith G. T., Moran T. H., Coyle J. T., Kuhar M. J., O'Donahue T. L., McHugh P. R. Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am J Physiol. 1984 Jan;246(1 Pt 2):R127–R130. doi: 10.1152/ajpregu.1984.246.1.R127. [DOI] [PubMed] [Google Scholar]


