Abstract
1 The pharmacological properties of CGP 37849 (DL-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid; 4-methyl-APPA) and its carboxyethylester, CGP 39551, novel unsaturated analogues of the N-methyl-D-aspartate (NMDA) receptor antagonist, 2-amino-5-phosphonopentanoate (AP5), were evaluated in rodent brain in vitro and in vivo.
2 Radioligand binding experiments demonstrated that CGP 37849 potently (Ki 220nM) and competitively inhibited NMDA-sensitive L-[3H]-glutamate binding to postsynaptic density (PSD) fractins from rat brain. It inhibited the binding of the selective NMDA receptor antagonist, [3H]-(±)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonate (CPP), with a Ki of 35nM, and was 4, 5 and 7 fold more potent than the antagonists ((±)-cis-4-phosphonomethylpiperidine-2-carboxylic acid) (CGS 19755), CPP and D-AP5, respectively. Inhibitory activity was associated exclusively with the trans configuration of the APPA molecule and with the D-stereoisomer. CGP 39551 showed weaker activity at NMDA receptor recognition sites and both compounds were weak or inactive at 18 other receptor binding sites.
3 CGP 37849 and CGP 39551 were inactive as inhibitors of L-[3H]-glutamate uptake into rat brain synaptosomes and had no effect on the release of endogenous glutamate from rat hippocampal slices evoked by electrical field stimulation.
4 In the hippocampal slice in vitro, CGP 37849 selectively and reversibly antagonized NMDA-evoked increases in CA1 pyramidal cell firing rate. In slices bathed in medium containing low Mg2+ levels, concentrations of CGP 37849 upto 10 μM suppressed burst firing evoked in CA1 neurones by stimulation of Schaffer collateral-commissural fibres without affecting the magnitude of the initial population spike; CGP 39551 exerted the same effect but was weaker. In vivo, oral administration to rats of either CGP 37849 or CGP 39551 selectively blocked firing in hippocampal neurones induced by ionophoretically-applied NMDA, without affecting the responses to quisqualate or kainate.
5 CGP 37849 and CGP 39551 suppressed maximal electroshock-induced seizures in mice with ED50S of 21 and 4 mgkg-1 p.o., respectively.
6 CGP 37849 and CGP 39551 are potent and competitive NMDA receptor antagonists which show significant central effects following oral administration to animals. As such, they may find value as tools to elucidate the roles of NMDA receptors in brain function, and potentially as therapeutic agents for the treatment of neurological disorders such as epilepsy and ischaemic brain damage in man.
Full text
PDF

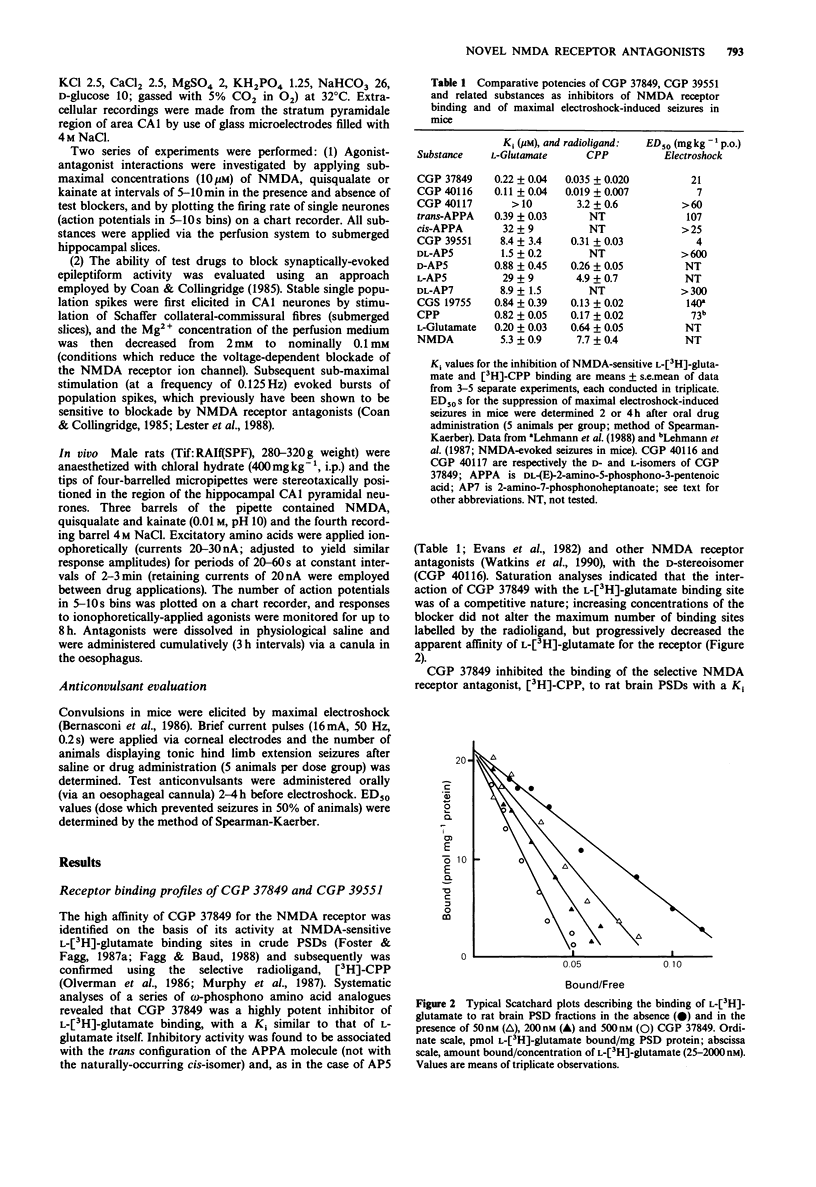
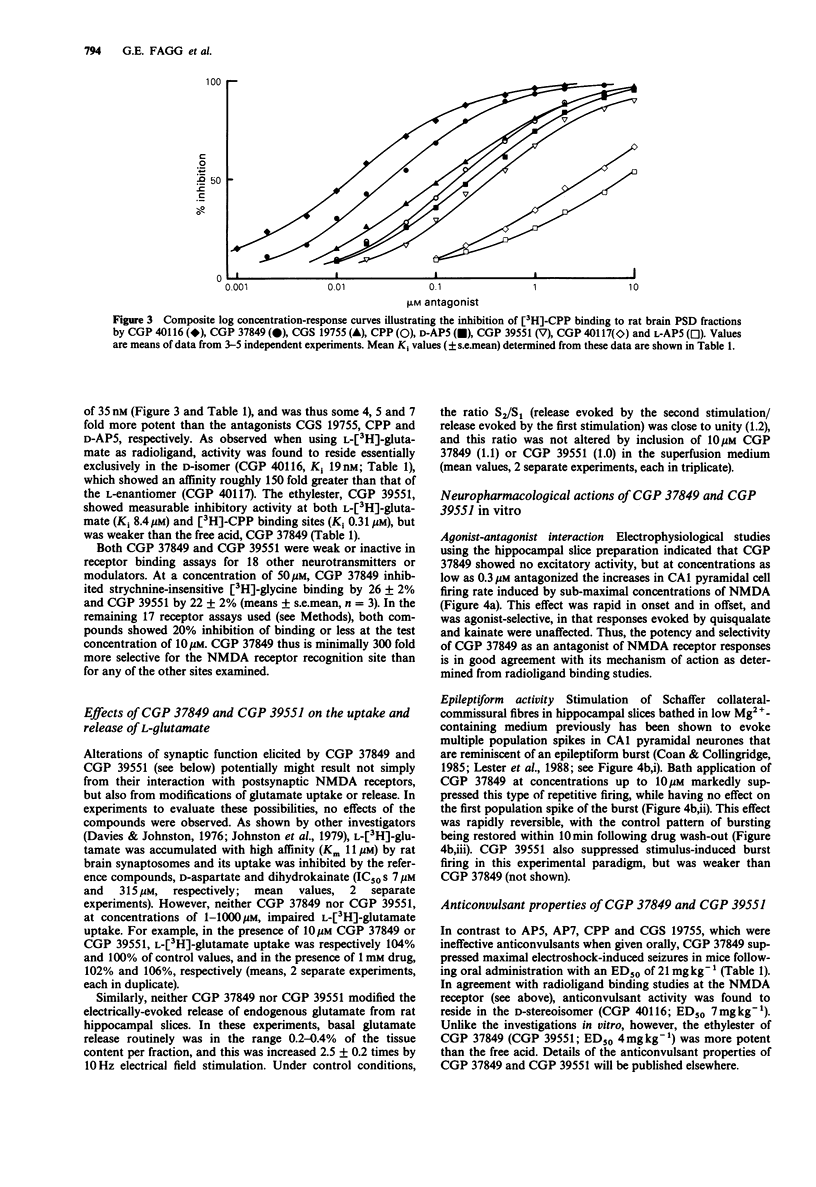
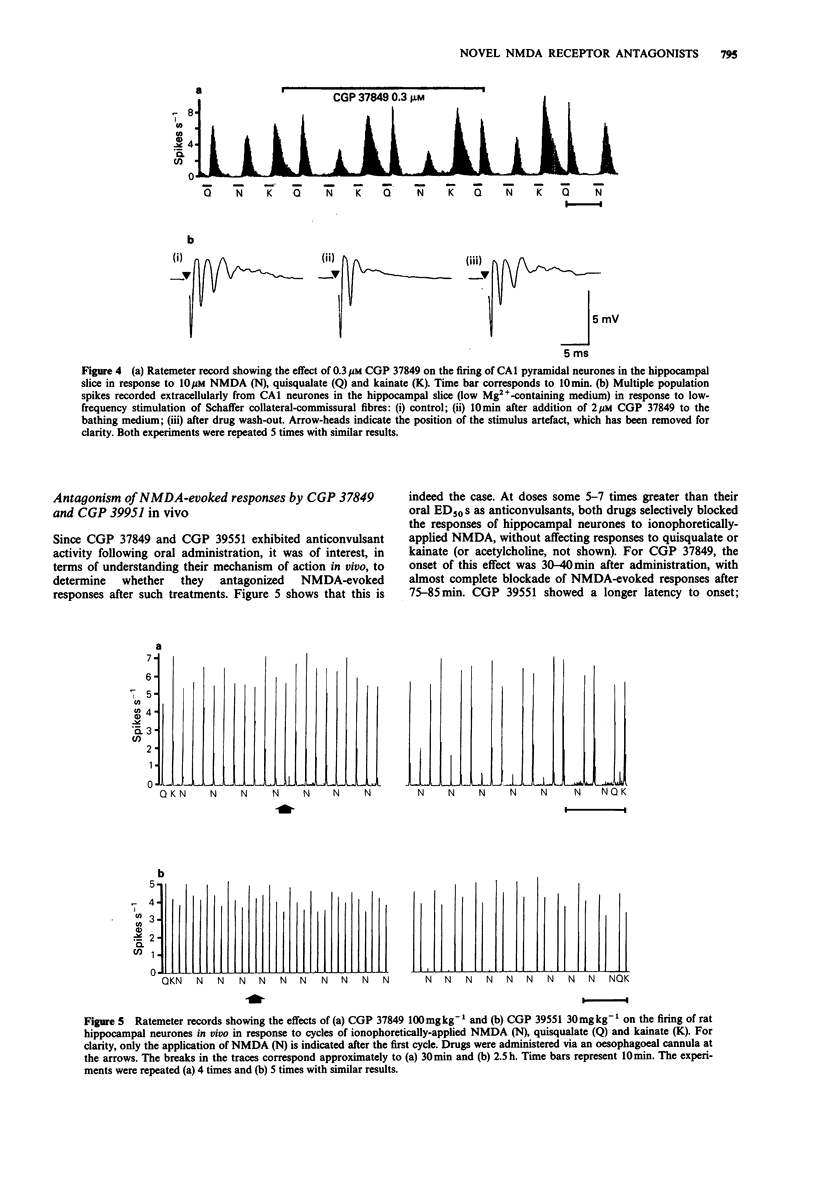


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers G. W., Goldberg M. P., Choi D. W. N-methyl-D-aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989 Apr;25(4):398–403. doi: 10.1002/ana.410250412. [DOI] [PubMed] [Google Scholar]
- Baud J., Thedinga K., Portet C., Schmutz M., Bittiger H., Bischoff S., Hauser K., Benedict M., Meier R., Fagg G. E. CGP 31358 binds to a site on the NMDA receptor that is coupled to both the transmitter recognition site and the channel domain. Neurosci Lett. 1989 Dec 15;107(1-3):184–188. doi: 10.1016/0304-3940(89)90814-8. [DOI] [PubMed] [Google Scholar]
- Bernasconi R., Jones R. S., Bittiger H., Olpe H. R., Heid J., Martin P., Klein M., Loo P., Braunwalder A., Schmutz M. Dose pipecolic acid interact with the central GABA-ergic system? J Neural Transm. 1986;67(3-4):175–189. doi: 10.1007/BF01243346. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Bowery N. G., Woodruff G. N. Light microscopic autoradiographic localisation of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur J Pharmacol. 1986 Jul 31;126(3):303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Collingridge G. L. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985 Jan 7;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Herrling P. L., Jones A. W., Olverman H. J., Pook P., Watkins J. C. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986 Sep 10;382(1):169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Johnston G. A. Uptake and release of D- and L-aspartate by rat brain slices. J Neurochem. 1976 May;26(5):1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkany J. W., Borosky S. A., Clissold D. B., Pontecorvo M. J. Dextromethorphan inhibits NMDA-induced convulsions. Eur J Pharmacol. 1988 Jun 22;151(1):151–154. doi: 10.1016/0014-2999(88)90707-8. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Comparison of L-[3H]glutamate, D-[3H]aspartate, DL-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol. 1987 Jan 20;133(3):291–300. doi: 10.1016/0014-2999(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Neurobiology. Taking apart NMDA receptors. Nature. 1987 Oct 1;329(6138):395–396. doi: 10.1038/329395a0. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Mena E. E., Monaghan D. T., Cotman C. W. Synaptic localization of kainic acid binding sites. Nature. 1981 Jan 1;289(5793):73–75. doi: 10.1038/289073a0. [DOI] [PubMed] [Google Scholar]
- France C. P., Woods J. H., Ornstein P. The competitive N-methyl-D-aspartate (NMDA) antagonist CGS 19755 attenuates the rate-decreasing effects of NMDA in rhesus monkeys without producing ketamine-like discriminative stimulus effects. Eur J Pharmacol. 1989 Jan 10;159(2):133–139. doi: 10.1016/0014-2999(89)90697-3. [DOI] [PubMed] [Google Scholar]
- Honoré T., Nielsen M. Complex structure of quisqualate-sensitive glutamate receptors in rat cortex. Neurosci Lett. 1985 Feb 28;54(1):27–32. doi: 10.1016/s0304-3940(85)80113-0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Twitchin B. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J Neurochem. 1979 Jan;32(1):121–127. doi: 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Leander J. D., Rathbun R. C., Zimmerman D. M. Anticonvulsant effects of phencyclidine-like drugs: relation to N-methyl-D-aspartic acid antagonism. Brain Res. 1988 Jun 28;454(1-2):368–372. doi: 10.1016/0006-8993(88)90839-6. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Hutchison A. J., McPherson S. E., Mondadori C., Schmutz M., Sinton C. M., Tsai C., Murphy D. E., Steel D. J., Williams M. CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988 Jul;246(1):65–75. [PubMed] [Google Scholar]
- Lehmann J., Schneider J., McPherson S., Murphy D. E., Bernard P., Tsai C., Bennett D. A., Pastor G., Steel D. J., Boehm C. CPP, a selective N-methyl-D-aspartate (NMDA)-type receptor antagonist: characterization in vitro and in vivo. J Pharmacol Exp Ther. 1987 Mar;240(3):737–746. [PubMed] [Google Scholar]
- Meldrum B. Possible therapeutic applications of antagonists of excitatory amino acid neurotransmitters. Clin Sci (Lond) 1985 Feb;68(2):113–122. doi: 10.1042/cs0680113. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. E., Schneider J., Boehm C., Lehmann J., Williams M. Binding of [3H]3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid to rat brain membranes: a selective, high-affinity ligand for N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1987 Mar;240(3):778–784. [PubMed] [Google Scholar]
- Olpe H. R., Lynch G. S. The action of piracetam on the electrical activity of the hippocampal slice preparation: a field potential analysis. Eur J Pharmacol. 1982 Jun 4;80(4):415–419. doi: 10.1016/0014-2999(82)90088-7. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Monaghan D. T., Cotman C. W., Watkins J. C. [3H]CPP, a new competitive ligand for NMDA receptors. Eur J Pharmacol. 1986 Nov 12;131(1):161–162. doi: 10.1016/0014-2999(86)90533-9. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Singh L., Oles R. J., Preston C., Iversen S. D. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989 Aug 11;167(1):127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- Waldmeier P. C., Wicki P., Feldtrauer J. J., Baumann P. A. Potential involvement of a baclofen-sensitive autoreceptor in the modulation of the release of endogenous GABA from rat brain slices in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1988 Mar;337(3):289–295. doi: 10.1007/BF00168841. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunger L. M., Fowler P. J., Zarevics P., Setler P. E. Novel inhibitors of gamma-aminobutyric acid (GABA) uptake: anticonvulsant actions in rats and mice. J Pharmacol Exp Ther. 1984 Jan;228(1):109–115. [PubMed] [Google Scholar]


