Abstract
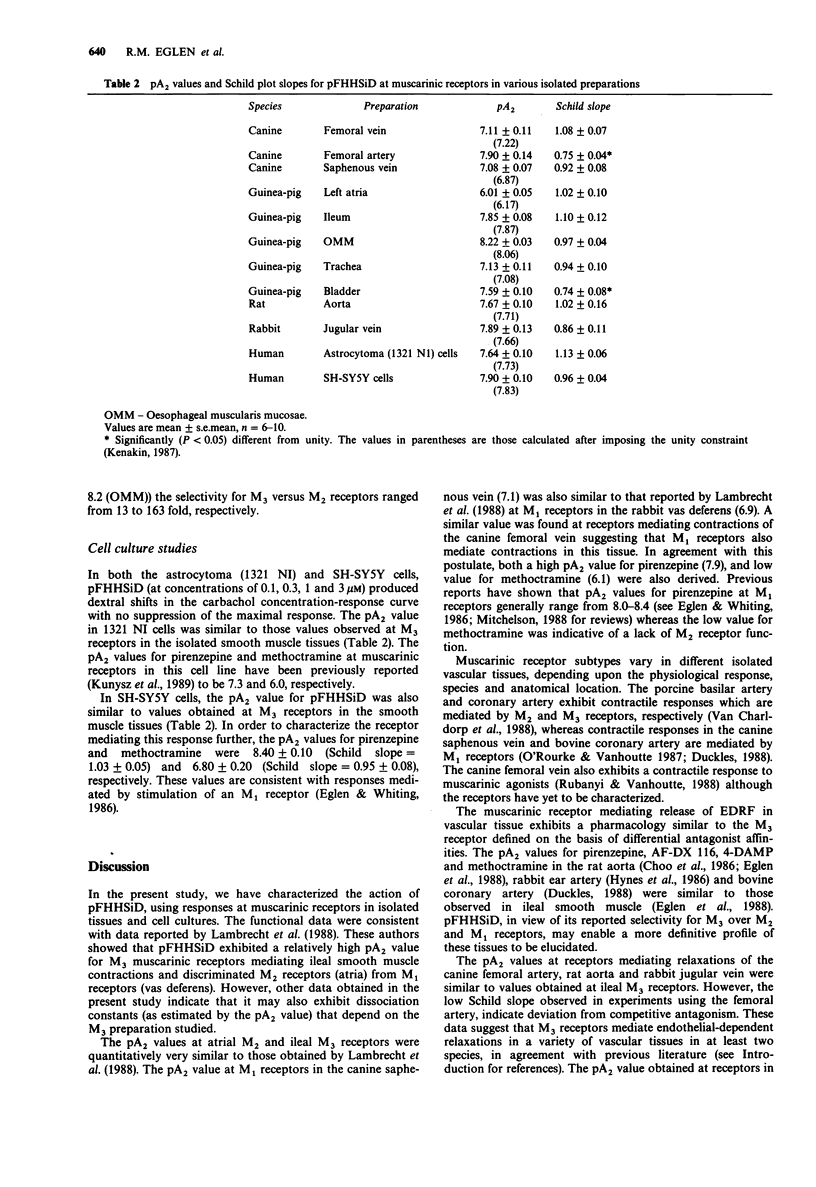
1. The antagonistic actions of parafluorohexahydrosiladiphenidol (pFHHSiD) at muscarinic receptors has been studied in cardiac muscle, smooth muscle and cell culture preparations. In this paper, the classification scheme of Doods et al. (1987) is employed. This scheme is based upon differential affinities of muscarinic antagonists. pFHHSiD exhibited high pA2 values at M3 receptors mediating contractions of guinea-pig ileum and oesophageal muscularis mucosae (7.8 and 8.2 respectively) whereas low values were determined at M2 receptors mediating negative inotropic responses in guinea-pig atria (6.0). Intermediate pA2 values were determined at M1 receptors mediating contractions of the canine femoral and saphenous veins. 2. The pA2 values of pFHHSiD at receptors mediating endothelial-dependent relaxation of rat aortic rings, rabbit jugular vein and canine femoral artery (7.6-7.9) were similar to those determined on the ileum. However, the pA2 values of pFHHSiD at receptors mediating contractions of the guinea-pig trachea (7.1), which has been previously shown to possess M3 receptors, were different from those determined in the ileum. 3. The similarity in pA2 values of pFHHSiD between the M3 receptors in guinea-pig ileum and the receptors mediating endothelial-dependent relaxations provide further evidence for the role of M3 receptors in this vascular response. Taken together, pA2 values for pFHHSiD range from 7.1 to 8.2, depending upon the M3 preparation used. The selectivity of the compound therefore for the M3 versus the M2 muscarinic receptor ranged from 13 to 163 fold.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Burston K. N., Vis A. Three types of muscarinic receptors? [proceedings]. Br J Pharmacol. 1980 Jan;68(1):141P–142P. [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A. Molecular neurobiology. Separating receptor subtypes from their shadows. Nature. 1988 Sep 22;335(6188):301–302. doi: 10.1038/335301a0. [DOI] [PubMed] [Google Scholar]
- Blinks J. R. Convenient apparatus for recording contractions of isolated heart muscle. J Appl Physiol. 1965 Jul;20(4):755–757. doi: 10.1152/jappl.1965.20.4.755. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Fatherazi S., Garthwaite J., White R. D. Muscarinic receptors in rat sympathetic ganglia. Br J Pharmacol. 1980 Dec;70(4):577–592. doi: 10.1111/j.1476-5381.1980.tb09777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Brann M. R. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988 Dec;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Choo L. K., Malta E., Mitchelson F. The affinity of some selective muscarinic receptor antagonists for the muscarinic receptor mediating endothelial-dependent relaxation of the rabbit and rat thoracic aorta. J Pharm Pharmacol. 1986 Nov;38(11):843–845. doi: 10.1111/j.2042-7158.1986.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Clague R. U., Eglen R. M., Strachan A. C., Whiting R. L. Action of agonists and antagonists at muscarinic receptors present on ileum and atria in vitro. Br J Pharmacol. 1985 Sep;86(1):163–170. doi: 10.1111/j.1476-5381.1985.tb09446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H. N., Mathy M. J., Davidesko D., van Charldorp K. J., de Jonge A., van Zwieten P. A. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987 Jul;242(1):257–262. [PubMed] [Google Scholar]
- Duckles S. P. Vascular muscarinic receptors: pharmacological characterization in the bovine coronary artery. J Pharmacol Exp Ther. 1988 Sep;246(3):929–934. [PubMed] [Google Scholar]
- Eglen R. M., Montgomery W. W., Dainty I. A., Dubuque L. K., Whiting R. L. The interaction of methoctramine and himbacine at atrial, smooth muscle and endothelial muscarinic receptors in vitro. Br J Pharmacol. 1988 Dec;95(4):1031–1038. doi: 10.1111/j.1476-5381.1988.tb11736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Comparison of the muscarinic receptors of the guinea-pig oesophageal muscularis mucosae and trachea in vitro. J Auton Pharmacol. 1988 Sep;8(3):181–189. doi: 10.1111/j.1474-8673.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Competitive and non-competitive antagonism exhibited by 'selective' antagonists at atrial and ileal muscarinic receptor subtypes. Br J Pharmacol. 1987 Apr;90(4):701–707. doi: 10.1111/j.1476-5381.1987.tb11223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Hynes M. R., Banner W., Jr, Yamamura H. I., Duckles S. P. Characterization of muscarinic receptors of the rabbit ear artery smooth muscle and endothelium. J Pharmacol Exp Ther. 1986 Jul;238(1):100–105. [PubMed] [Google Scholar]
- Kamikawa Y., Uchida K., Shimo Y. Heterogeneity of muscarinic receptors in the guinea pig esophageal muscularis mucosae and ileal longitudinal muscle. Gastroenterology. 1985 Mar;88(3):706–716. doi: 10.1016/0016-5085(85)90141-6. [DOI] [PubMed] [Google Scholar]
- Kunysz E. A., Michel A. D., Whiting R. L., Woods K. The human astrocytoma cell line 1321 N1 contains M2-glandular type muscarinic receptors linked to phosphoinositide turnover. Br J Pharmacol. 1989 Feb;96(2):271–278. doi: 10.1111/j.1476-5381.1989.tb11813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Forth B., Strohmann C., Tacke R., Mutschler E. p-fluoro-hexahydro-sila-difenidol: the first M2 beta-selective muscarinic antagonist. Eur J Pharmacol. 1988 Jul 26;152(1-2):193–194. doi: 10.1016/0014-2999(88)90856-4. [DOI] [PubMed] [Google Scholar]
- Leff P., Martin G. R. The classification of 5-hydroxytryptamine receptors. Med Res Rev. 1988 Apr-Jun;8(2):187–202. doi: 10.1002/med.2610080203. [DOI] [PubMed] [Google Scholar]
- McCaig D. J., De Jonckheere B. Effect of cromakalim on bronchoconstriction evoked by cholinergic nerve stimulation in guinea-pig isolated trachea. Br J Pharmacol. 1989 Oct;98(2):662–668. doi: 10.1111/j.1476-5381.1989.tb12641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorre C., Cassinelli A., Quaglia W. Differential blockade of muscarinic receptor subtypes by polymethylene tetraamines. Novel class of selective antagonists of cardiac M-2 muscarinic receptors. J Med Chem. 1987 Jan;30(1):201–204. doi: 10.1021/jm00384a034. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Delmendo R., Stefanich E., Whiting R. L. Binding characteristics of the muscarinic receptor subtype of the NG108-15 cell line. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):62–67. doi: 10.1007/BF00169208. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Methoctramine reveals heterogeneity of M2 muscarinic receptors in longitudinal ileal smooth muscle membranes. Eur J Pharmacol. 1988 Jan 19;145(3):305–311. doi: 10.1016/0014-2999(88)90434-7. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Johnson R. D. Characterization of alpha-1 adrenergic receptors linked to [3H]inositol metabolism in rat cerebral cortex. J Pharmacol Exp Ther. 1984 Aug;230(2):317–323. [PubMed] [Google Scholar]
- Mitchelson F. Muscarinic receptor differentiation. Pharmacol Ther. 1988;37(3):357–423. doi: 10.1016/0163-7258(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Nilvebrant L., Sparf B. Receptor binding profiles of some selective muscarinic antagonists. Eur J Pharmacol. 1988 Jun 22;151(1):83–96. doi: 10.1016/0014-2999(88)90695-4. [DOI] [PubMed] [Google Scholar]
- O'Rourke S. T., Vanhoutte P. M. Subtypes of muscarinic receptors on adrenergic nerves and vascular smooth muscle of the canine saphenous vein. J Pharmacol Exp Ther. 1987 Apr;241(1):64–67. [PubMed] [Google Scholar]
- Parker R. B., Waud D. R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J Pharmacol Exp Ther. 1971 Apr;177(1):1–12. [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Roffel A. F., Elzinga C. R., Van Amsterdam R. G., De Zeeuw R. A., Zaagsma J. Muscarinic M2 receptors in bovine tracheal smooth muscle: discrepancies between binding and function. Eur J Pharmacol. 1988 Aug 9;153(1):73–82. doi: 10.1016/0014-2999(88)90589-4. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Heterogeneity of endothelium-dependent responses to acetylcholine in canine femoral arteries and veins. Separation of the role played by endothelial and smooth muscle cells. Blood Vessels. 1988;25(2):75–81. [PubMed] [Google Scholar]
- Serra M., Mei L., Roeske W. R., Lui G. K., Watson M., Yamamura H. I. The intact human neuroblastoma cell (SH-SY5Y) exhibits high-affinity [3H]pirenzepine binding associated with hydrolysis of phosphatidylinositols. J Neurochem. 1988 May;50(5):1513–1521. doi: 10.1111/j.1471-4159.1988.tb03038.x. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Van Charldorp K. J., Davidesko D., Van Zwieten P. A. Selectivity of methoctramine for muscarinic receptors in porcine cerebral arteries. Eur J Pharmacol. 1988 May 20;150(1-2):197–199. doi: 10.1016/0014-2999(88)90770-4. [DOI] [PubMed] [Google Scholar]


