Abstract
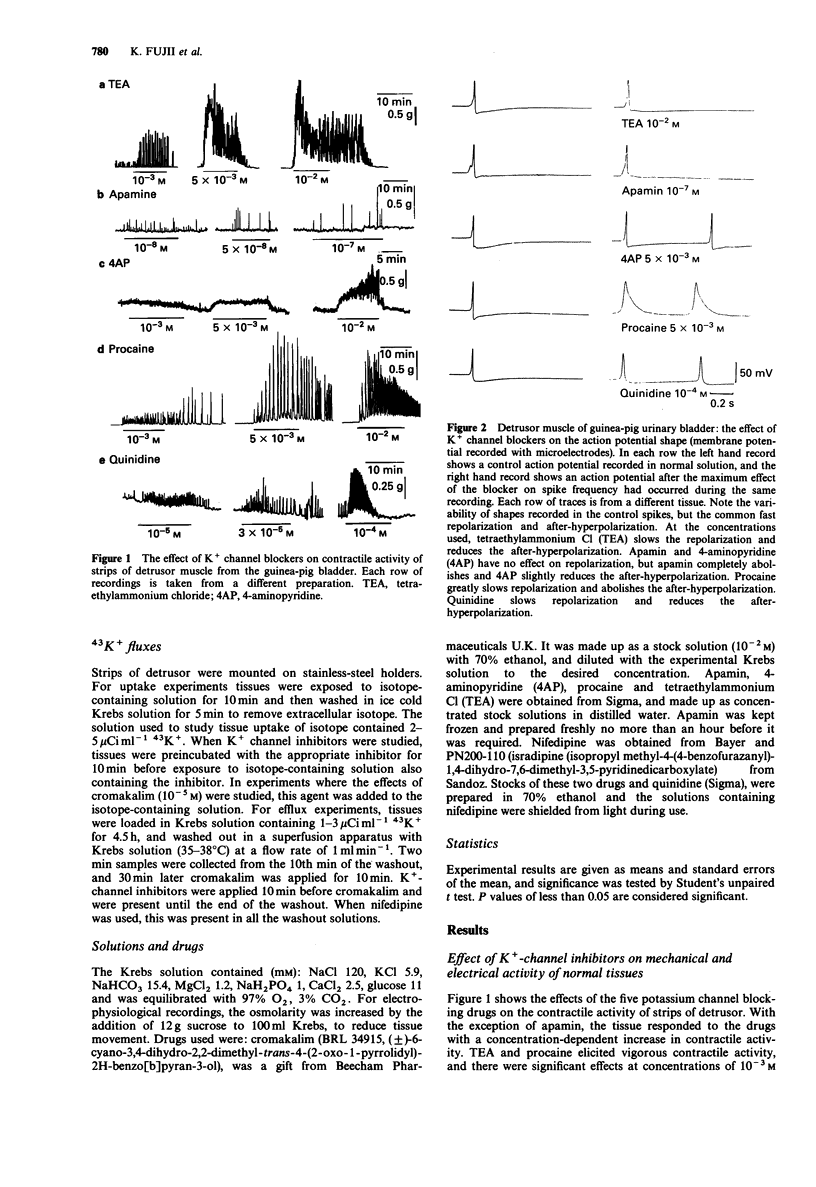
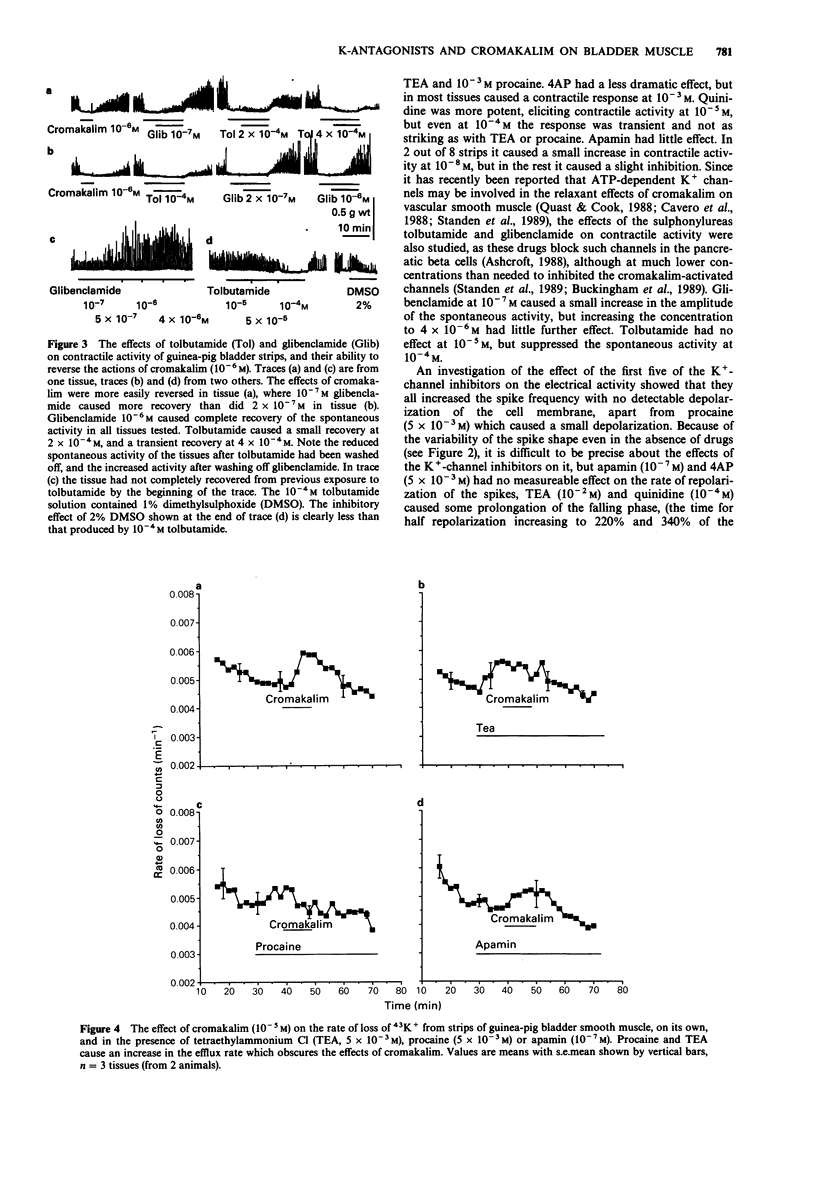
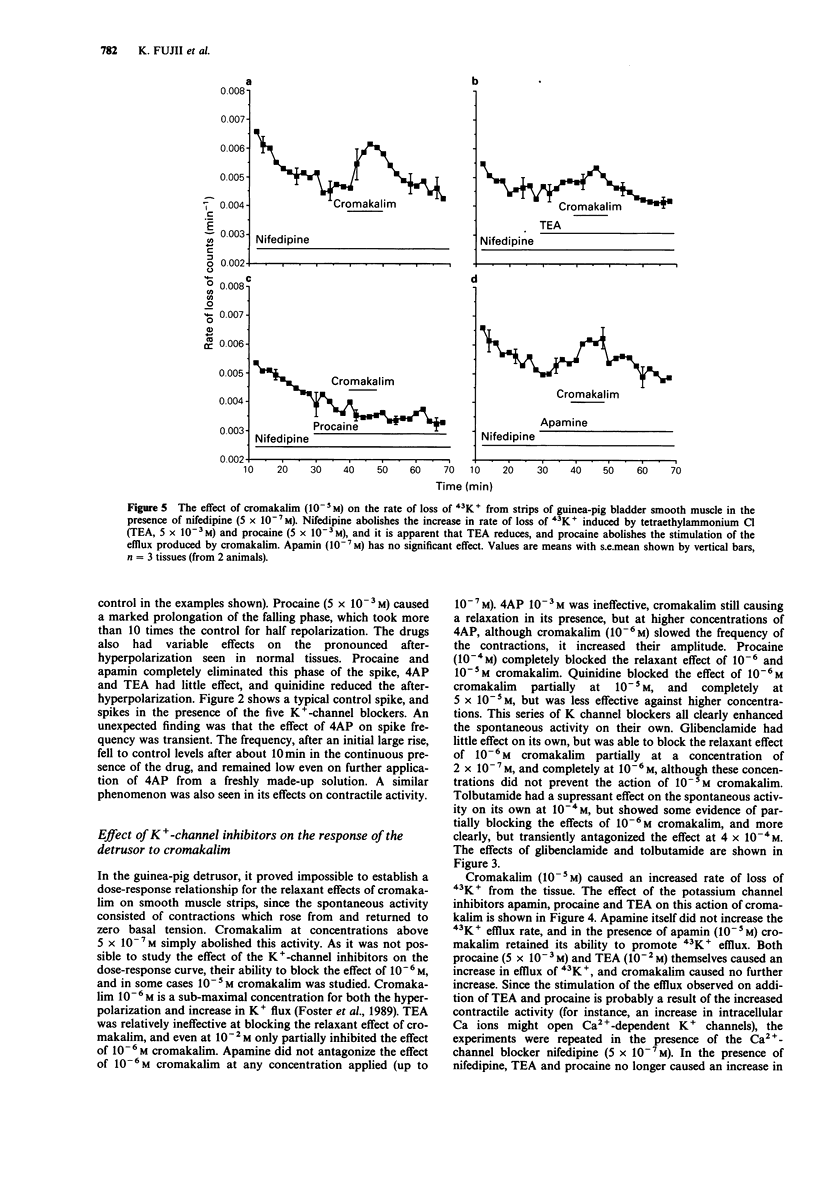
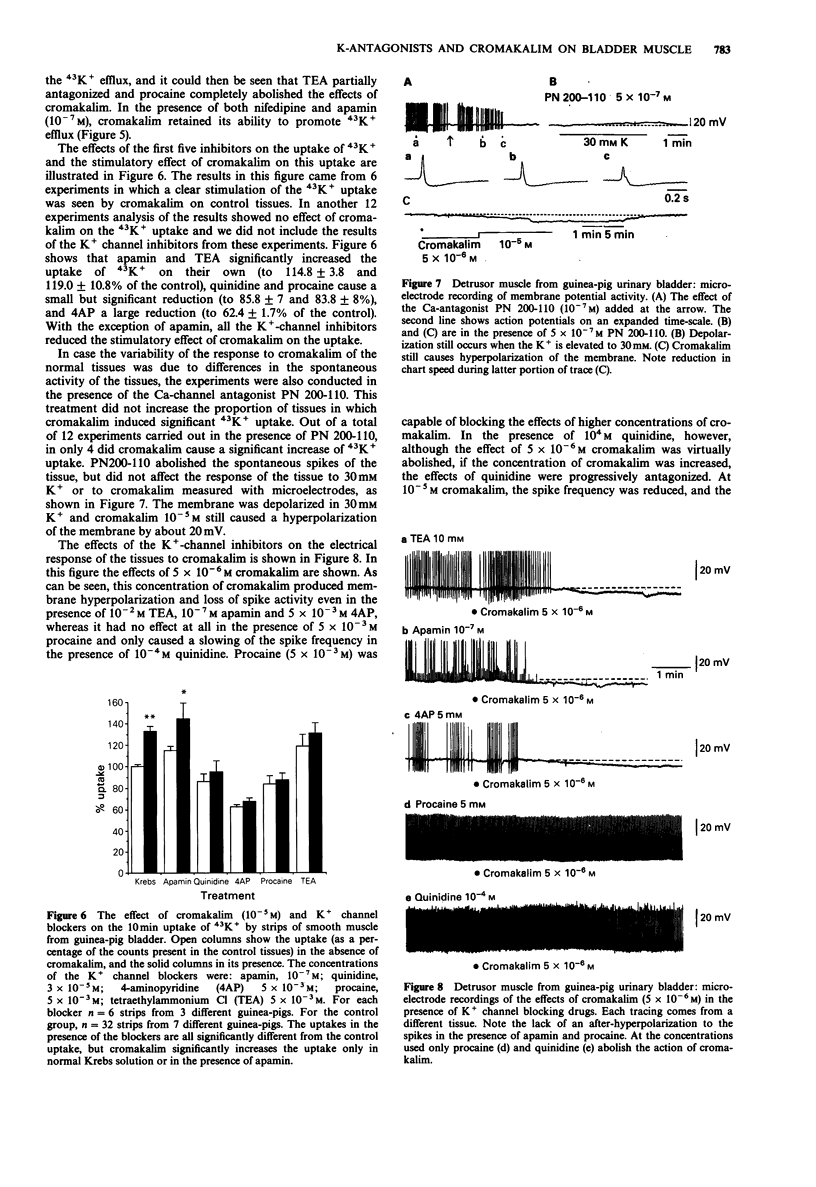
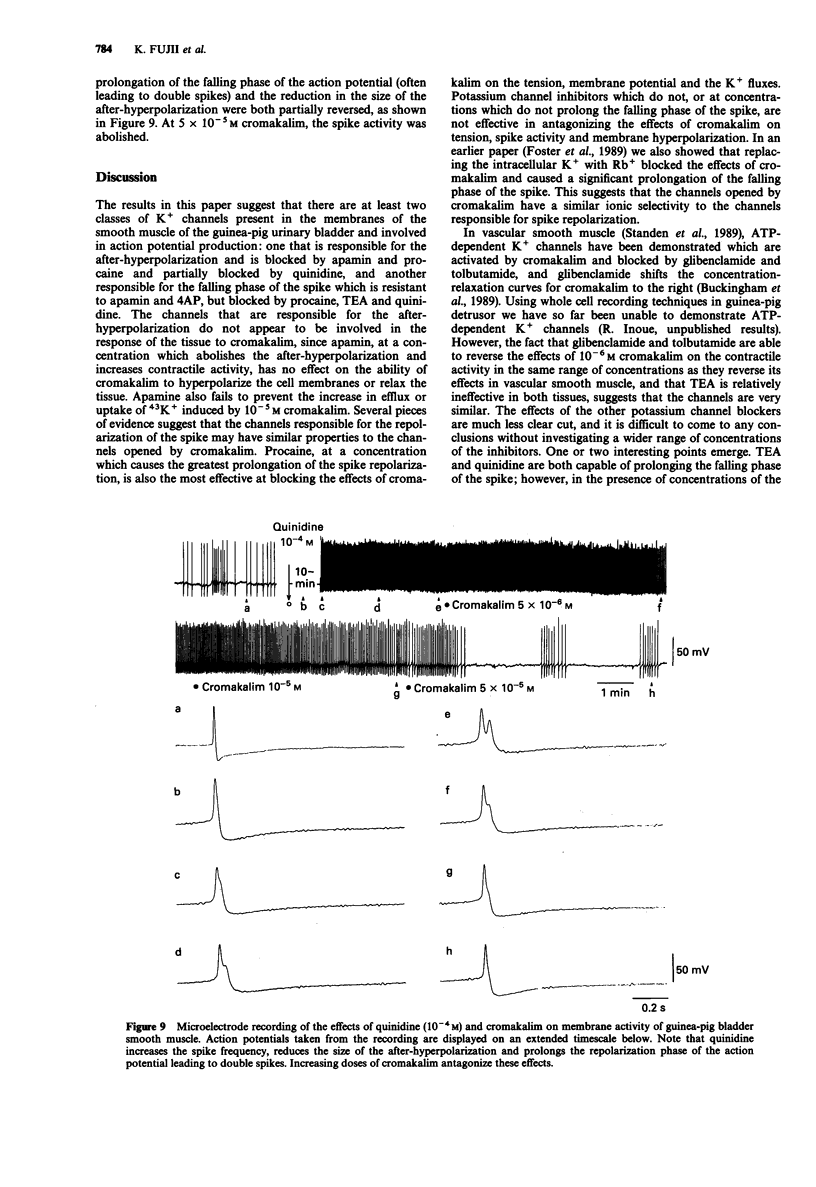
1. The K+ channel blocking drugs tetraethylammonium Cl (TEA), procaine, 4-aminopyridine (4AP) and quinidine all produced concentration-dependent contractions of strips of smooth muscle from the guinea-pig urinary bladder. Apamin and glibenclamide caused little increase in the mechanical activity, and tolbutamide inhibited it. 2. TEA, procaine, 4AP, quinidine and apamin all increased the frequencies of spontaneous action potentials recorded with microelectrodes. TEA, quinidine and procaine all caused prolongation of the falling phase of the spike, and procaine and apamin completely abolished the after-hyperpolarization. 3. TEA and procaine increased K+ efflux from the tissue, an effect blocked by nifedipine. TEA and apamin increased, whereas quinidine, procaine and 4AP decreased K+ uptake. 4. Cromakalim caused a concentration-dependent hyperpolarization of the membrane, abolished spike activity, increased K+ fluxes and relaxed the smooth muscle. The relaxant effect of cromakalim was unaffected by apamin, and in its presence the effects of cromakalim on membrane potential and K+ fluxes were unchanged. Procaine abolished all the effects of cromakalim, and TEA at high concentrations reduced but did not abolish these effects. Quinidine reduced the effects of cromakalim on tension and membrane potential, but its actions were surmounted by higher concentrations of cromakalim. The effects of 4AP on tension and membrane properties were transitory, but it had some effects on the actions of cromakalim. Glibenclamide and tolbutamide reversed the relaxant effects of submaximal cromakalim concentrations, tolbutamide only transiently. 5. It is concluded that the channels opened by cromakalim are not those involved in generating the spike after-hyperpolarization.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Buckingham R. E., Hamilton T. C., Howlett D. R., Mootoo S., Wilson C. Inhibition by glibenclamide of the vasorelaxant action of cromakalim in the rat. Br J Pharmacol. 1989 May;97(1):57–64. doi: 10.1111/j.1476-5381.1989.tb11923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan S. M., Creed K. E. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol. 1981 Oct;74(2):353–358. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan S. M., Creed K. E. Non-cholinergic neurotransmission and the effects of peptides on the urinary bladder of guinea-pigs and rabbits. J Physiol. 1986 May;374:103–115. doi: 10.1113/jphysiol.1986.sp016068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Creed K. E. Effects of ions and drugs on the smooth muscle cell membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):127–141. doi: 10.1007/BF00586905. [DOI] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):115–126. doi: 10.1007/BF00586904. [DOI] [PubMed] [Google Scholar]
- Foster C. D., Fujii K., Kingdon J., Brading A. F. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol. 1989 May;97(1):281–291. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Creed K. E. Changes in the membrane potential of the smooth muscle cells of the guinea pig urinary bladder in various environments. Jpn J Physiol. 1972 Dec;22(6):667–683. doi: 10.2170/jjphysiol.22.667. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Neuroleptics antagonize a calcium-activated potassium channel in airway smooth muscle. J Gen Physiol. 1987 Feb;89(2):339–352. doi: 10.1085/jgp.89.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]


