Abstract
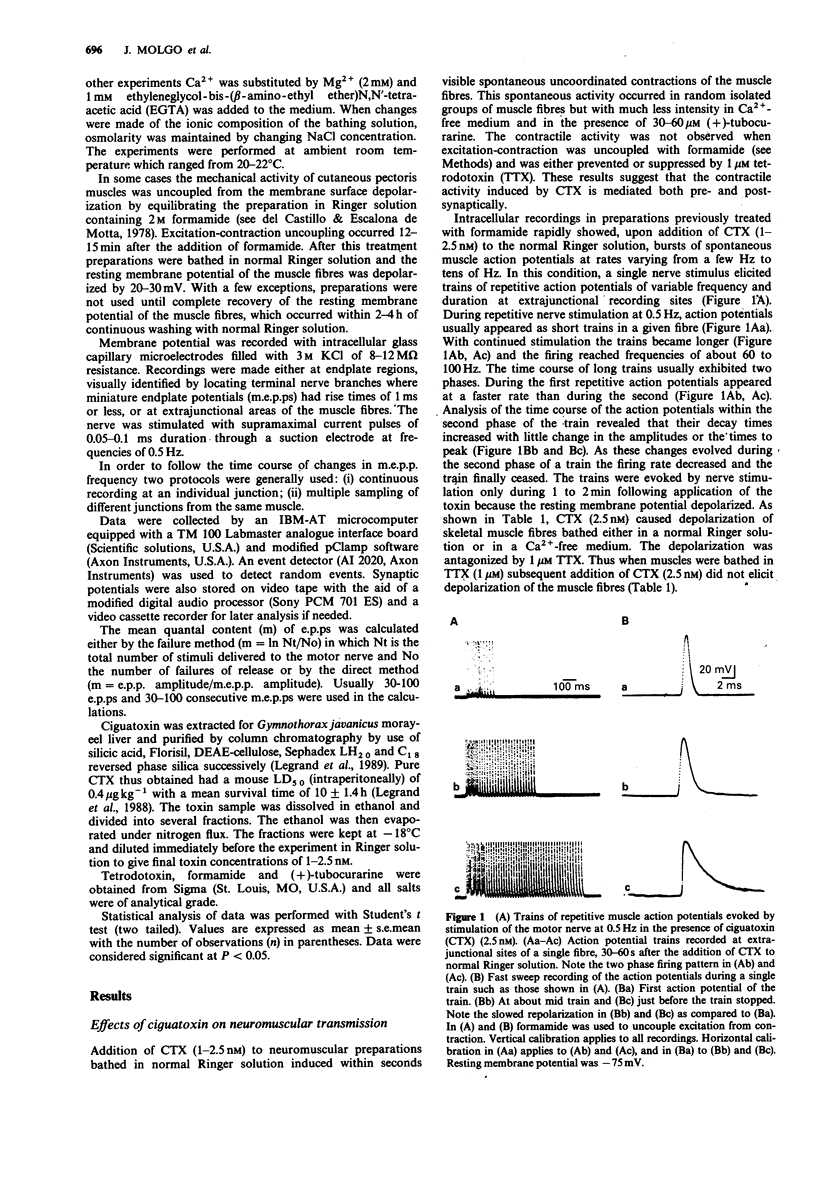
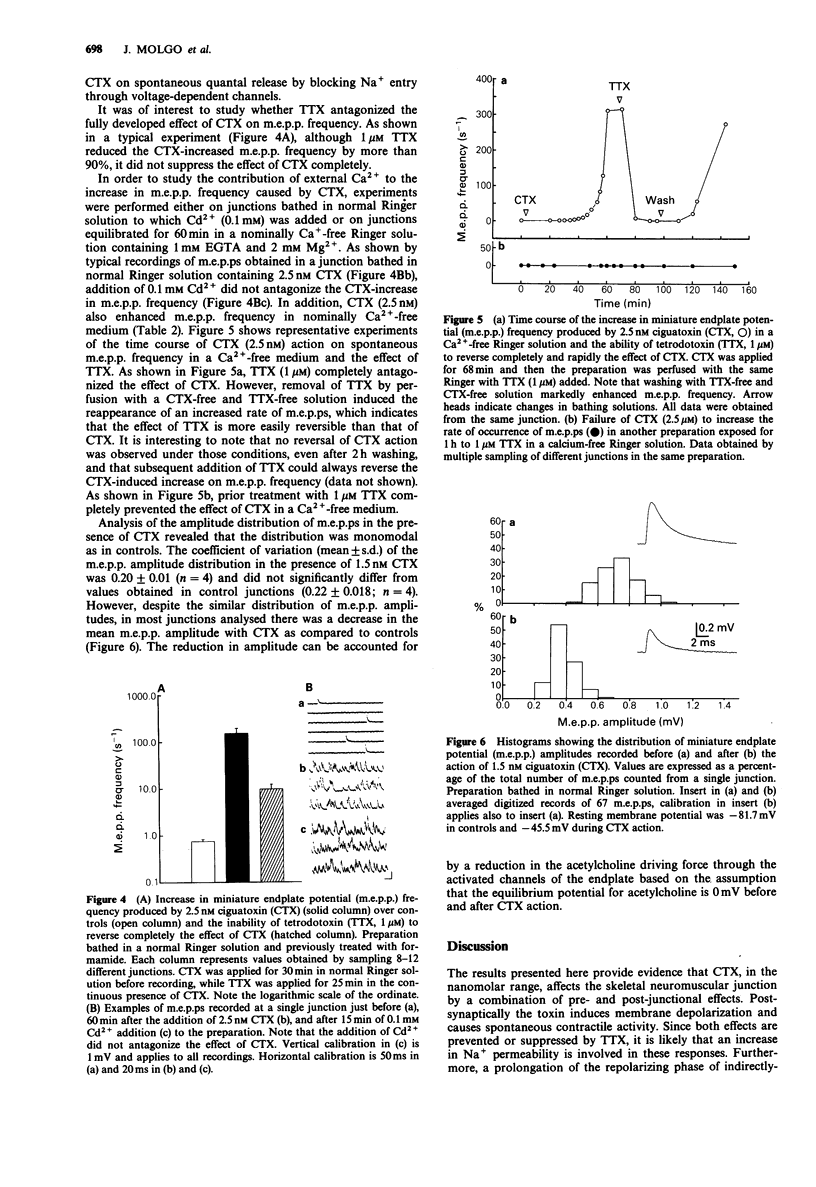
1. Ciguatoxin (CTX), a marine toxin produced by the benthic dinoflagellate Gambierdiscus toxicus, is responsible for a complex endemic disease in man known as ciguatera fish poisoning. In the present study we have investigated the effects of purified CTX extracted for Gymnothorax javanicus moray-eel liver on frog isolated neuromuscular preparations with conventional electrophysiological techniques. 2. CTX (1-2.5 nM) applied to cutaneous pectoris nerve-muscle preparations induced, after a short delay, spontaneous fibrillations of the muscle fibres that could be suppressed with 1 microM tetrodotoxin (TTX) or by formamide to uncouple excitation-contraction. 3. In preparations treated with formamide, CTX (1-2.5 nM) caused either spontaneous or repetitive muscle action potentials (up to frequencies of 60-100 Hz) in response to a single nerve stimulus. Recordings performed at extrajunctional regions of the muscle membrane revealed that during the repetitive firing a prolongation of the repolarizing phase of the action potential occurred. At junctional sites the repetitive action potentials were triggered by repetitive endplate potentials (e.p.ps). 4. CTX (2.5 nM) caused a TTX-sensitive depolarization of the muscle membrane. 5. In junctions equilibrated in solutions containing high Mg2+ + low Ca2+, addition of CTX (1.5 nM) first induced an average increase of 239 +/- 36% in the mean quantal content of e.p.ps. Subsequently CTX reduced and finally blocked nerve-evoked transmitter release irreversibly. 6. CTX (1.5-2.5 nM) increased the frequency of miniature endplate potentials (m.e.p.ps) in junctions bathed either in normal Ringer, low Ca2(+)-high Mg2+ medium or in a nominally Ca2(+)-free solution containing EGTA.2+ Extensive washing with toxin-free solutions did not reverse the effect.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L. Effects of the potassium channel blocking dendrotoxins on acetylcholine release and motor nerve terminal activity. Br J Pharmacol. 1988 Jan;93(1):215–221. doi: 10.1111/j.1476-5381.1988.tb11424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D., Luke V. S., Narahashi T., Vogel S. M. Nerve membrane sodium channels as the target site of brevetoxins at neuromuscular junctions. Br J Pharmacol. 1986 Dec;89(4):731–738. doi: 10.1111/j.1476-5381.1986.tb11177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnis R. Clinical aspects of ciguatera (fish poisoning) in French Polynesia. Hawaii Med J. 1968 Sep-Oct;28(1):25–28. [PubMed] [Google Scholar]
- Bagnis R., Kuberski T., Laugier S. Clinical observations on 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg. 1979 Nov;28(6):1067–1073. doi: 10.4269/ajtmh.1979.28.1067. [DOI] [PubMed] [Google Scholar]
- Benoit E., Legrand A. M., Dubois J. M. Effects of ciguatoxin on current and voltage clamped frog myelinated nerve fibre. Toxicon. 1986;24(4):357–364. doi: 10.1016/0041-0101(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Bidard J. N., Vijverberg H. P., Frelin C., Chungue E., Legrand A. M., Bagnis R., Lazdunski M. Ciguatoxin is a novel type of Na+ channel toxin. J Biol Chem. 1984 Jul 10;259(13):8353–8357. [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J., Miller D. M., Tindall D. R. Interactions of maitotoxin with voltage-sensitive calcium channels in cultured neuronal cells. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4582–4585. doi: 10.1073/pnas.81.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y. Y., Quastel D. M., Saint D. A. Multiple actions of cadmium on transmitter release at the mouse neuromuscular junction. Can J Physiol Pharmacol. 1987 Oct;65(10):2131–2136. doi: 10.1139/y87-334. [DOI] [PubMed] [Google Scholar]
- Gusovsky F., Hollingsworth E. B., Daly J. W. Regulation of phosphatidylinositol turnover in brain synaptoneurosomes: stimulatory effects of agents that enhance influx of sodium ions. Proc Natl Acad Sci U S A. 1986 May;83(9):3003–3007. doi: 10.1073/pnas.83.9.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Tesseraux I. Effects of sea-anemone toxin (ATX-II) on the frequency of miniature endplate potentials at rat neuromuscular junctions. Br J Pharmacol. 1984 Apr;81(4):573–574. doi: 10.1111/j.1476-5381.1984.tb16120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Khan A. R., Lemeignan M., Molgo J. Effects of toxin II from the sea anemone Anemonia sulcata on contractile and electrical responses of frog skeletal muscle fibres. Toxicon. 1986;24(4):373–384. doi: 10.1016/0041-0101(86)90197-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Login I. S., Yasumoto T. Maitotoxin activates quantal transmitter release at the neuromuscular junction: evidence for elevated intraterminal Ca2+ in the motor nerve terminal. Brain Res. 1985 Nov 4;346(2):357–362. doi: 10.1016/0006-8993(85)90870-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ohizumi Y., Yasumoto T. The mechanism of action of maitotoxin in relation to Ca2+ movements in guinea-pig and rat cardiac muscles. Br J Pharmacol. 1985 Oct;86(2):385–391. doi: 10.1111/j.1476-5381.1985.tb08907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Login I. S., Judd A. M., Cronin M. J., Koike K., Schettini G., Yasumoto T., MacLeod R. M. The effects of maitotoxin on 45Ca2+ flux and hormone release in GH3 rat pituitary cells. Endocrinology. 1985 Feb;116(2):622–627. doi: 10.1210/endo-116-2-622. [DOI] [PubMed] [Google Scholar]
- Lombet A., Bidard J. N., Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987 Jul 27;219(2):355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Lowe D. A., Richardson N. P., Taylor P., Donatsch P. Increasing intracellular sodium triggers calcium release from bound pools. Nature. 1976 Mar 25;260(5549):337–338. doi: 10.1038/260337a0. [DOI] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Effects of guanidine on transmitter release and neuronal excitability. J Physiol. 1977 Mar;266(1):69–89. doi: 10.1113/jphysiol.1977.sp011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinek R., Lev-Tov A., Meiri H., Erulkar S. D., Rahamimoff R. Regulatory role of intracellular sodium ions in neurotransmitter secretion. Isr J Med Sci. 1982 Jan;18(1):37–43. [PubMed] [Google Scholar]
- Miyamoto T., Ohizumi Y., Washio H., Yasumoto Y. Potent excitatory effect of maitotoxin on Ca channels in the insect skeletal muscle. Pflugers Arch. 1984 Apr;400(4):439–441. doi: 10.1007/BF00587546. [DOI] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Tazieff-Depierre F. Enhancement by Anemonia sulcata toxin II of spontaneous quantal transmitter release from mammalian motor nerve terminals. Toxicon. 1986;24(5):441–450. doi: 10.1016/0041-0101(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Molgó J., Mallart A. Effects of Anemonia sulcata toxin II on presynaptic currents and evoked transmitter release at neuromuscular junctions of the mouse. Pflugers Arch. 1985 Dec;405(4):349–353. doi: 10.1007/BF00595687. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Schettini G., Koike K., Login I. S., Judd A. M., Cronin M. J., Yasumoto T., MacLeod R. M. Maitotoxin stimulates hormonal release and calcium flux in rat anterior pituitary cells in vitro. Am J Physiol. 1984 Oct;247(4 Pt 1):E520–E525. doi: 10.1152/ajpendo.1984.247.4.E520. [DOI] [PubMed] [Google Scholar]
- Scheuer P. J., Takahashi W., Tsutsumi J., Yoshida T. Ciguatoxin: isolation and chemical nature. Science. 1967 Mar 10;155(3767):1267–1268. doi: 10.1126/science.155.3767.1267. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tatsumi M., Ohizumi Y., Yasumoto T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J Biol Chem. 1983 Sep 25;258(18):10944–10949. [PubMed] [Google Scholar]
- Withers N. W. Ciguatera fish poisoning. Annu Rev Med. 1982;33:97–111. doi: 10.1146/annurev.me.33.020182.000525. [DOI] [PubMed] [Google Scholar]
- del Castillo J., Escalona de Motta G. A new method for excitation-contraction uncoupling in frog skeletal muscle. J Cell Biol. 1978 Sep;78(3):782–784. doi: 10.1083/jcb.78.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]


