Abstract
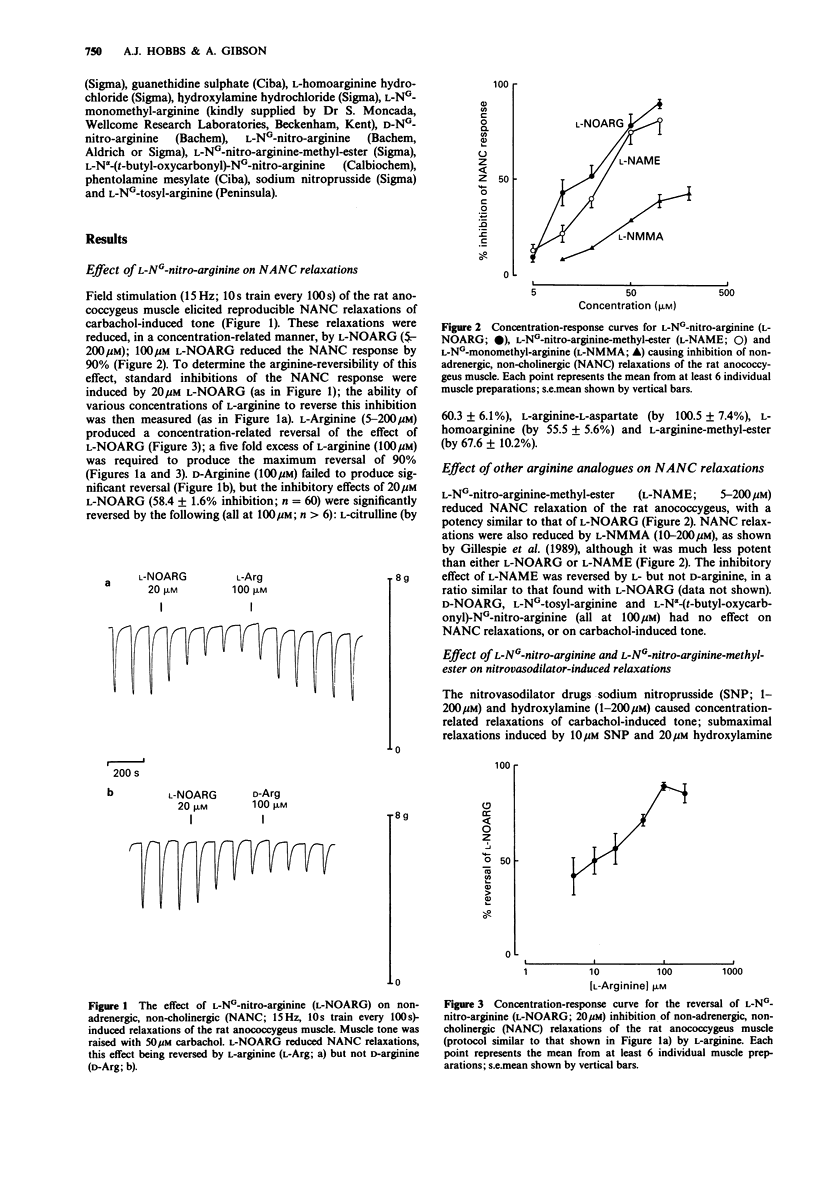
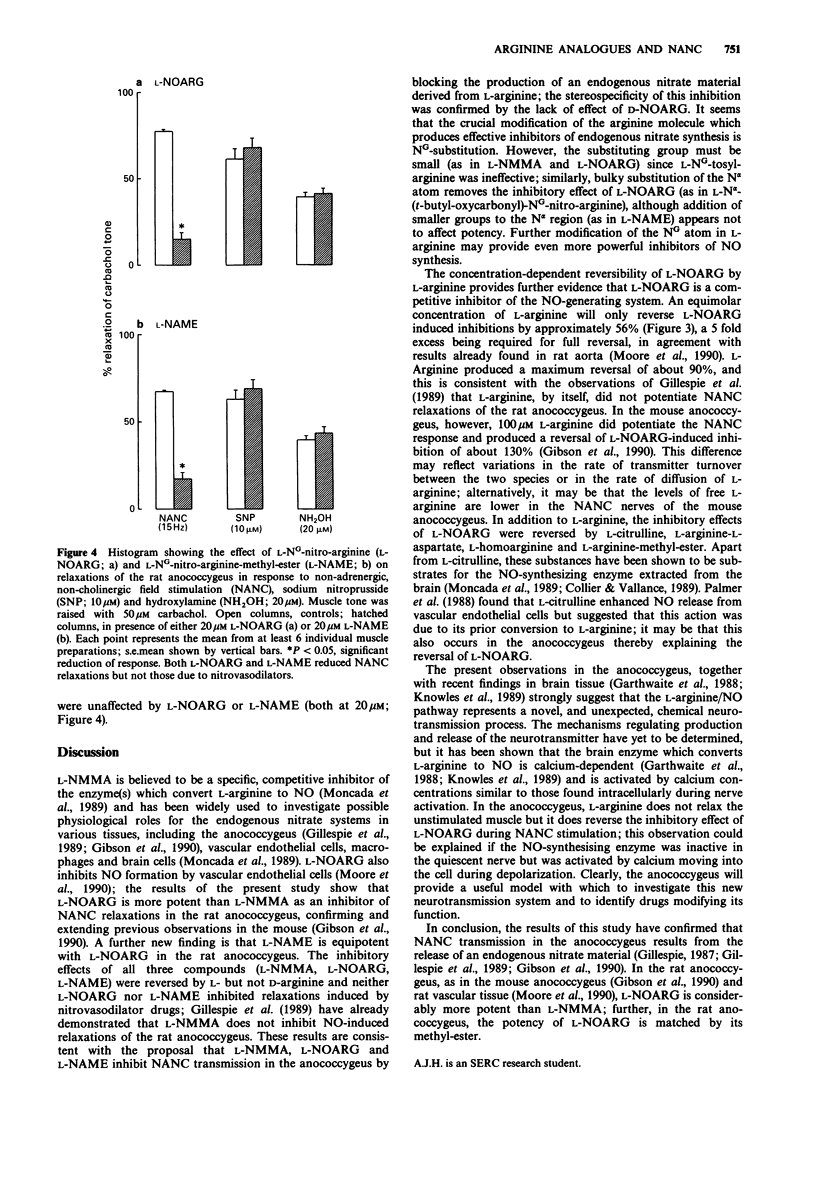
1. The effects of L-NG-nitro-arginine (L-NOARG) and some other arginine analogues on non-adrenergic, non-cholinergic (NANC) relaxations of the rat anococcygeus muscle were investigated. 2. L-NOARG (5-200 microM) produced concentration-related inhibition of the NANC response; 100 microM L-NOARG produced 90% inhibition. 3. L-Arginine (5-200 microM) produced a concentration-related reversal of the inhibitory effect of 20 microM L-NOARG; a five fold excess of L-arginine (100 microM) was required to obtain the maximum reversal of 90%. D-Arginine (100 microM) produced no such reversal, but significant reversal was produced by L-citrulline, L-arginine-L-aspartate, L-homoarginine and L-arginine-methyl-ester (all at 100 microM). 4. L-NG-nitro-arginine-methyl-ester (L-NAME; 5-200 microM) also reduced NANC relaxations, with a potency similar to that of L-NOARG; both L-NOARG and L-NAME were some ten times more potent than L-NG-monomethyl-arginine (L-NMMA). Like L-NOARG, the effects of L-NAME (20 microM) were reversed by 100 microM L- but not D-arginine. 5. Neither L-NOARG nor L-NAME (both 20 microM) affected submaximal relaxations induced by 10 microM sodium nitroprusside or 20 microM hydroxylamine. 6. D-NOARG, L-NG-tosyl-arginine and L-N alpha-(t-butyl-oxycarbonyl)-NG-nitro-arginine (all at 100 microM) had no effect on NANC relaxations. 7. Thus, in the rat anococcygeus, L-NOARG and L-NAME are more potent than L-NMMA as prejunctional inhibitors of NANC transmission.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier J., Vallance P. Second messenger role for NO widens to nervous and immune systems. Trends Pharmacol Sci. 1989 Nov;10(11):427–431. doi: 10.1016/s0165-6147(89)80001-x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. Influence of haemoglobin and erythrocytes on the effects of EDRF, a smooth muscle inhibitory factor, and nitric oxide on vascular and non-vascular smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1151–1156. doi: 10.1111/j.1476-5381.1988.tb11750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Smith J. A., Lewis M. J., Henderson A. H. Evidence that inhibitory factor extracted from bovine retractor penis is nitrite, whose acid-activated derivative is stabilized nitric oxide. Br J Pharmacol. 1988 Mar;93(3):579–586. doi: 10.1111/j.1476-5381.1988.tb10313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]


