Abstract
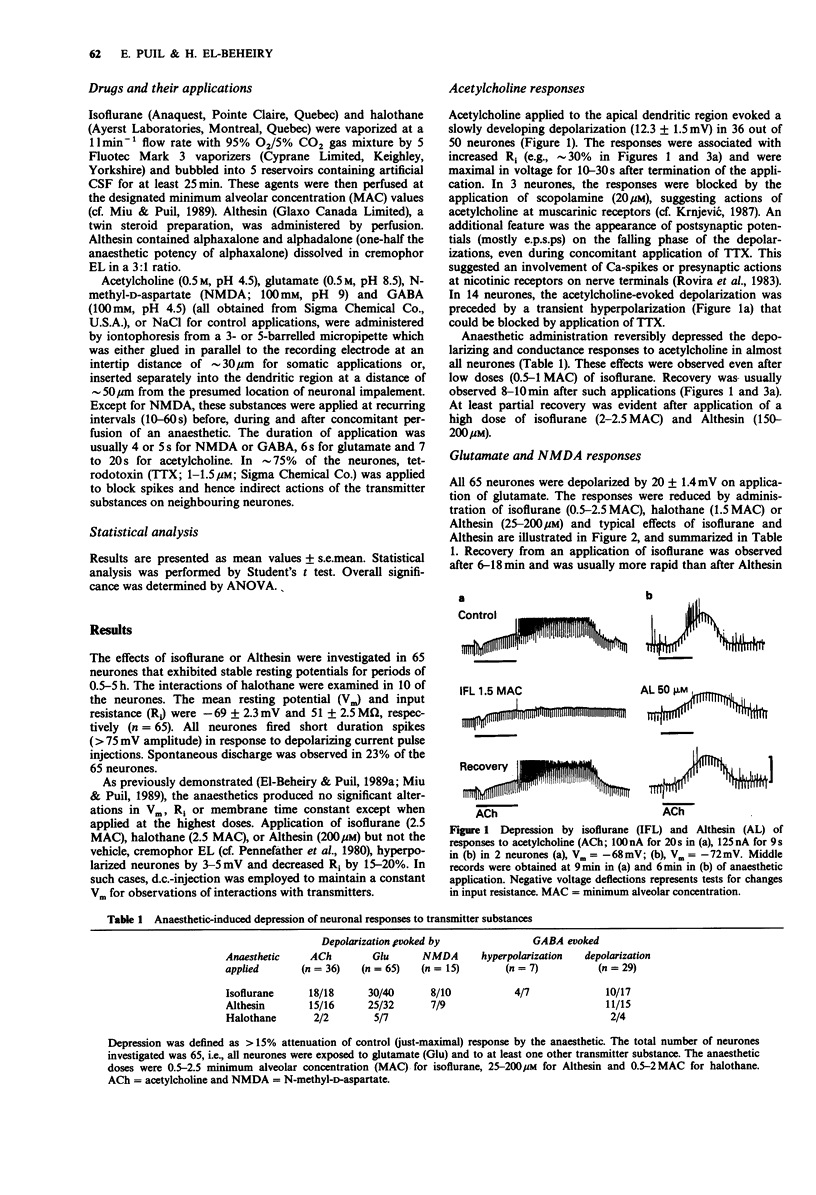
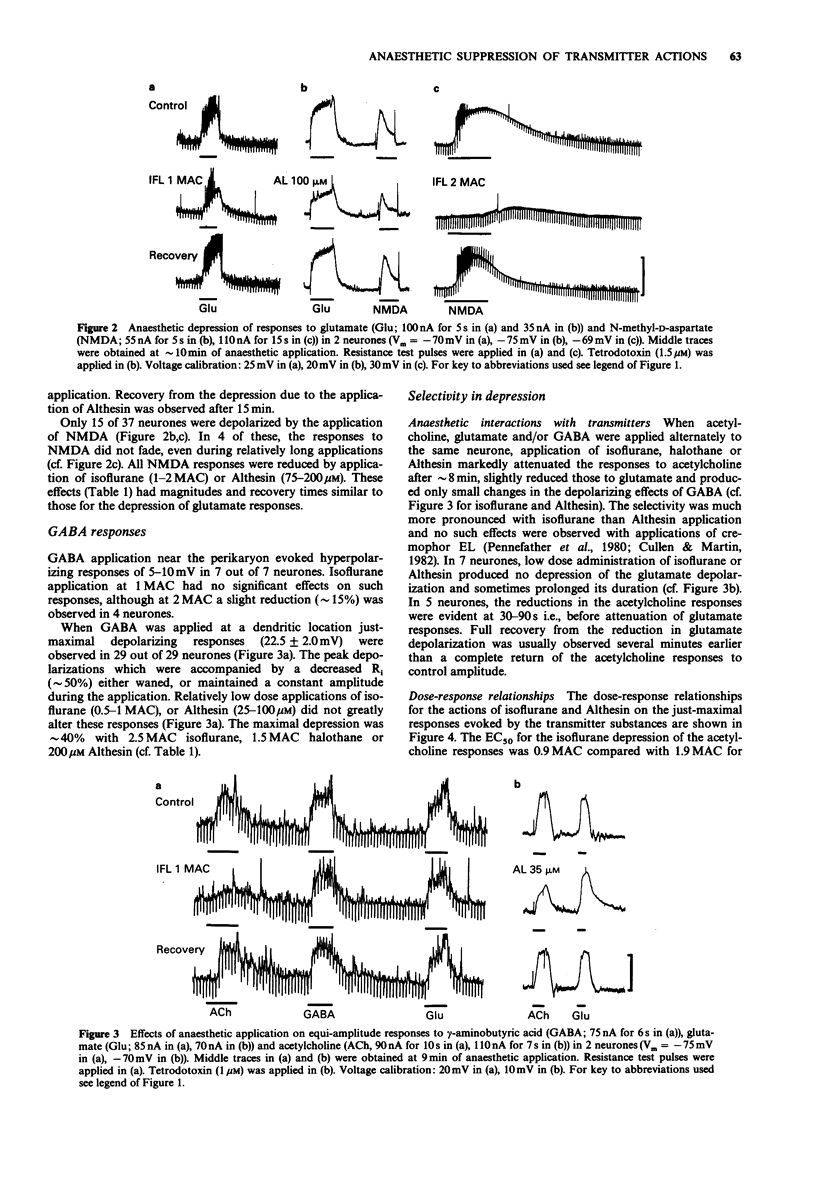
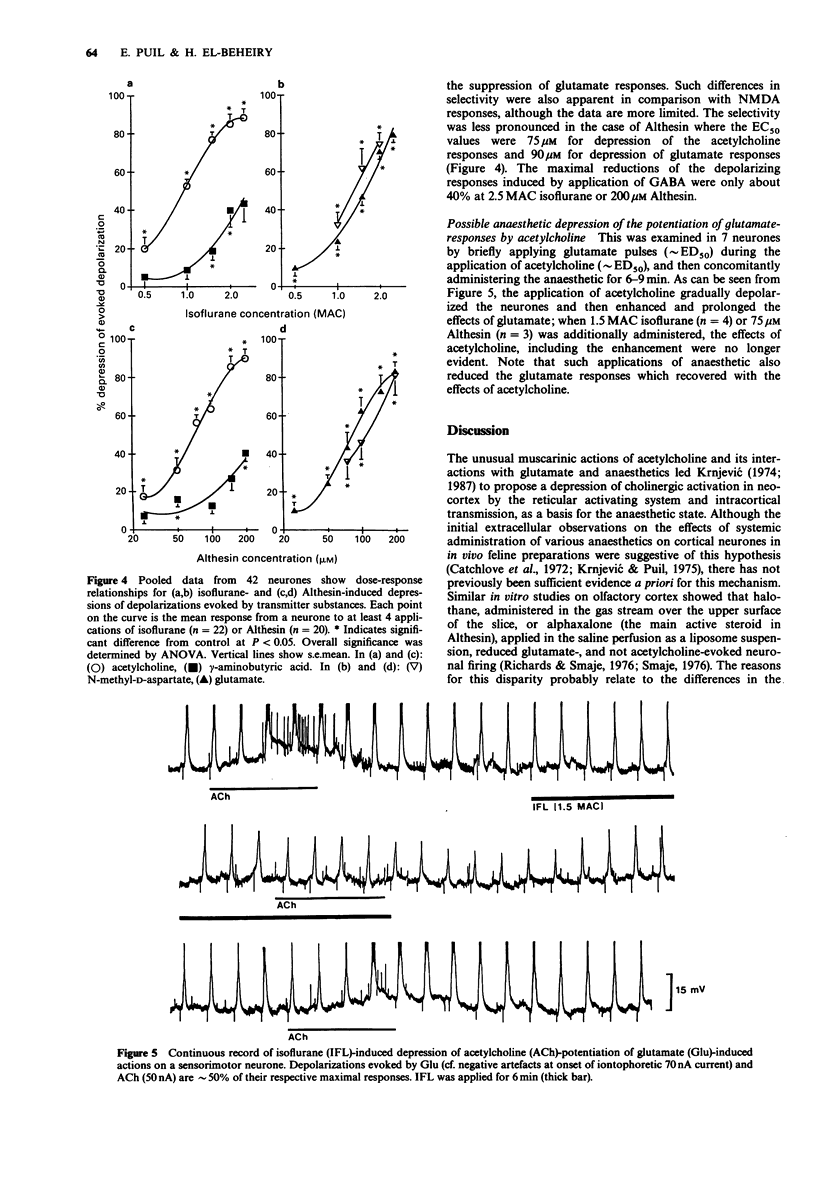
1. The effects of general anaesthetics were investigated on neuronal sensitivities to transmitter substances, which were determined by iontophoretic applications of acetylcholine, glutamate, N-methyl-D-aspartate (NMDA) and gamma-aminobutyrate (GABA) during intracellular recording in in vitro slice preparations of neocortex (guinea-pig). 2. In most of the 65 neurones studied, perfusion of isoflurane (0.5-2.5 minimum alveolar concentration (MAC)) or Althesin (25-200 microM) and, in some cases, halothane (0.5-2 MAC), markedly reduced the depolarizing responses and associated membrane conductance changes evoked by dendritic applications of acetylcholine, glutamate, NMDA and GABA. 3. The order of depression was acetylcholine greater than glutamate or NMDA much greater than GABA. This selectivity could also be assessed from the EC50 for the isoflurane-induced depression of the just-maximal responses to acetylcholine, which was 0.9 MAC compared with an EC50 = 1.9 MAC for the suppression of glutamate responses. The selectivity was less pronounced in the case of the actions of Althesin, where the EC50s were 75 microM for the depression of acetylcholine responses and 90 microM for the depression of glutamate responses. 4. The hyperpolarizing responses observed when GABA was applied near the perikaryon in 7 neurones, were slightly reduced (approximately 15%) in 4, and unchanged in 3 neurones during anaesthetic application. 5. The pronounced depression of the responsiveness to the putative arousal transmitters and an observed blockade of acetylcholine-induced potentiation of glutamate actions suggest that anaesthetics produce unconsciousness, at least in part, by interfering with subsynaptic mechanisms of neocortical activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura H., Ikemoto Y. Action of enflurane on cholinergic transmission in identified Aplysia neurones. Br J Pharmacol. 1986 Nov;89(3):573–582. doi: 10.1111/j.1476-5381.1986.tb11158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Harrison N. L., Lange G. D., Owen D. G. Potentiation of gamma-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987 May;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg-Johnsen J., Langmoen I. A. Isoflurane hyperpolarizes neurones in rat and human cerebral cortex. Acta Physiol Scand. 1987 Aug;130(4):679–685. doi: 10.1111/j.1748-1716.1987.tb08192.x. [DOI] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L. GABA responses in rat dentate granule neurons are mediated by chloride. Can J Physiol Pharmacol. 1988 May;66(5):637–642. doi: 10.1139/y88-099. [DOI] [PubMed] [Google Scholar]
- Catchlove R. F., Krnjević K., Maretić H. Similarity between effects of general anesthetics and dinitrophenol on cortical neurones. Can J Physiol Pharmacol. 1972 Nov;50(11):1111–1114. doi: 10.1139/y72-162. [DOI] [PubMed] [Google Scholar]
- Celesia G. G., Jasper H. H. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966 Nov;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Brunner E. A. Alteration of tricarboxylic acid cycle metabolism in rat brain slices by halothane. J Neurochem. 1978 Jun;30(6):1421–1430. doi: 10.1111/j.1471-4159.1978.tb10474.x. [DOI] [PubMed] [Google Scholar]
- Cullen K. D., Martin R. J. Dissimilar influences of some injectable anaesthetics on the responses of reticulo-spinal neurones to inhibitory transmitters in the lamprey. Br J Pharmacol. 1982 Nov;77(3):493–504. doi: 10.1111/j.1476-5381.1982.tb09323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison R. L., Jr, Anthony B. L., Narayanan T. K., Aronstam R. S. Effects of halothane on high affinity agonist binding and guanine nucleotide sensitivity of muscarinic acetylcholine receptors from brainstem of rat. Neuropharmacology. 1987 Aug;26(8):1201–1205. doi: 10.1016/0028-3908(87)90269-3. [DOI] [PubMed] [Google Scholar]
- Francesconi W., Müller C. M., Singer W. Cholinergic mechanisms in the reticular control of transmission in the cat lateral geniculate nucleus. J Neurophysiol. 1988 Jun;59(6):1690–1718. doi: 10.1152/jn.1988.59.6.1690. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Y., Akaike N., Ono K. Differential effects of enflurane on Glu- and ACH-induced chloride currents in Aplysia neurons. Life Sci. 1988;42(16):1557–1564. doi: 10.1016/0024-3205(88)90014-8. [DOI] [PubMed] [Google Scholar]
- JASPER H. H., KHAN R. T., ELLIOTT K. A. AMINO ACIDS RELEASED FROM THE CEREBRAL CORTEX IN RELATION TO ITS STATE OF ACTIVATION. Science. 1965 Mar 19;147(3664):1448–1449. doi: 10.1126/science.147.3664.1448. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E. Halothane suppresses slow inward currents in hippocampal slices. Can J Physiol Pharmacol. 1988 Dec;66(12):1570–1575. doi: 10.1139/y88-257. [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Flatman J. A. The interaction between barbiturate anaesthetics and excitatory amino acid responses on cat spinal neurones. Neuropharmacology. 1981 Mar;20(3):227–240. doi: 10.1016/0028-3908(81)90126-x. [DOI] [PubMed] [Google Scholar]
- Lysakowski A., Wainer B. H., Bruce G., Hersh L. B. An atlas of the regional and laminar distribution of choline acetyltransferase immunoreactivity in rat cerebral cortex. Neuroscience. 1989;28(2):291–336. doi: 10.1016/0306-4522(89)90180-2. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Miu P., Puil E. Isoflurane-induced impairment of synaptic transmission in hippocampal neurons. Exp Brain Res. 1989;75(2):354–360. doi: 10.1007/BF00247941. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Puil E., Quastel D. M. Steroid anaesthetics: inhibition of depolarization-secretion coupling at the mouse motor nerve terminal. Can J Physiol Pharmacol. 1980 Oct;58(10):1221–1228. doi: 10.1139/y80-185. [DOI] [PubMed] [Google Scholar]
- Puil E., Gimbarzevsky B. Modifications in membrane properties of trigeminal sensory neurons during general anesthesia. J Neurophysiol. 1987 Jul;58(1):87–104. doi: 10.1152/jn.1987.58.1.87. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Saint D. A. Modification of motor nerve terminal excitability by alkanols and volatile anaesthetics. Br J Pharmacol. 1986 Aug;88(4):747–756. doi: 10.1111/j.1476-5381.1986.tb16247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. Actions of general anaesthetics on synaptic transmission in the CNS. Br J Anaesth. 1983 Mar;55(3):201–207. doi: 10.1093/bja/55.3.201. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Smaje J. C. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976 Nov;58(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- Roth S. H., Williams P. J. The non-specific membrane binding properties of delta9-tetrahydrocannabinol and the effects of various solubilizers. J Pharm Pharmacol. 1979 Apr;31(4):224–230. doi: 10.1111/j.2042-7158.1979.tb13484.x. [DOI] [PubMed] [Google Scholar]
- Rovira C., Ben-Ari Y., Cherubini E., Krnjevic K., Ropert N. Pharmacology of the dendritic action of acetylcholine and further observations on the somatic disinhibition in the rat hippocampus in situ. Neuroscience. 1983 Jan;8(1):97–106. doi: 10.1016/0306-4522(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Savaki H. E., Desban M., Glowinski J., Besson M. J. Local cerebral glucose consumption in the rat. I. Effects of halothane anesthesia. J Comp Neurol. 1983 Jan 1;213(1):36–45. doi: 10.1002/cne.902130104. [DOI] [PubMed] [Google Scholar]
- Sawada S., Yamamoto C. Blocking action of pentobarbital on receptors for excitatory amino acids in the guinea pig hippocampus. Exp Brain Res. 1985;59(2):226–231. doi: 10.1007/BF00230901. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Sear J. W., Prys-Roberts C. Plasma concentrations of alphaxalone during continuous infusion of Althesin. Br J Anaesth. 1979 Sep;51(9):861–865. doi: 10.1093/bja/51.9.861. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Smaje J. C. General anaesthetics and the acetylcholine-sensitivity of cortical neurons. Br J Pharmacol. 1976 Nov;58(3):359–366. doi: 10.1111/j.1476-5381.1976.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenoshita M., Takahashi T. Mechanisms of halothane action on synaptic transmission in motoneurons of the newborn rat spinal cord in vitro. Brain Res. 1987 Feb 3;402(2):303–310. doi: 10.1016/0006-8993(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Young A. P., Sigman D. S. Allosteric effects of volatile anesthetics on the membrane-bound acetylcholine receptor protein. I. Stabilization of the high-affinity state. Mol Pharmacol. 1981 Nov;20(3):498–505. [PubMed] [Google Scholar]
- Zorychta E., Capek R. Depression of spinal monosynaptic transmission by diethyl ether: quantal analysis of unitary synaptic potentials. J Pharmacol Exp Ther. 1978 Dec;207(3):825–836. [PubMed] [Google Scholar]
- el-Beheiry H., Puil E. Anaesthetic depression of excitatory synaptic transmission in neocortex. Exp Brain Res. 1989;77(1):87–93. doi: 10.1007/BF00250570. [DOI] [PubMed] [Google Scholar]
- el-Beheiry H., Puil E. Postsynaptic depression induced by isoflurane and Althesin in neocortical neurons. Exp Brain Res. 1989;75(2):361–368. doi: 10.1007/BF00247942. [DOI] [PubMed] [Google Scholar]


