Abstract
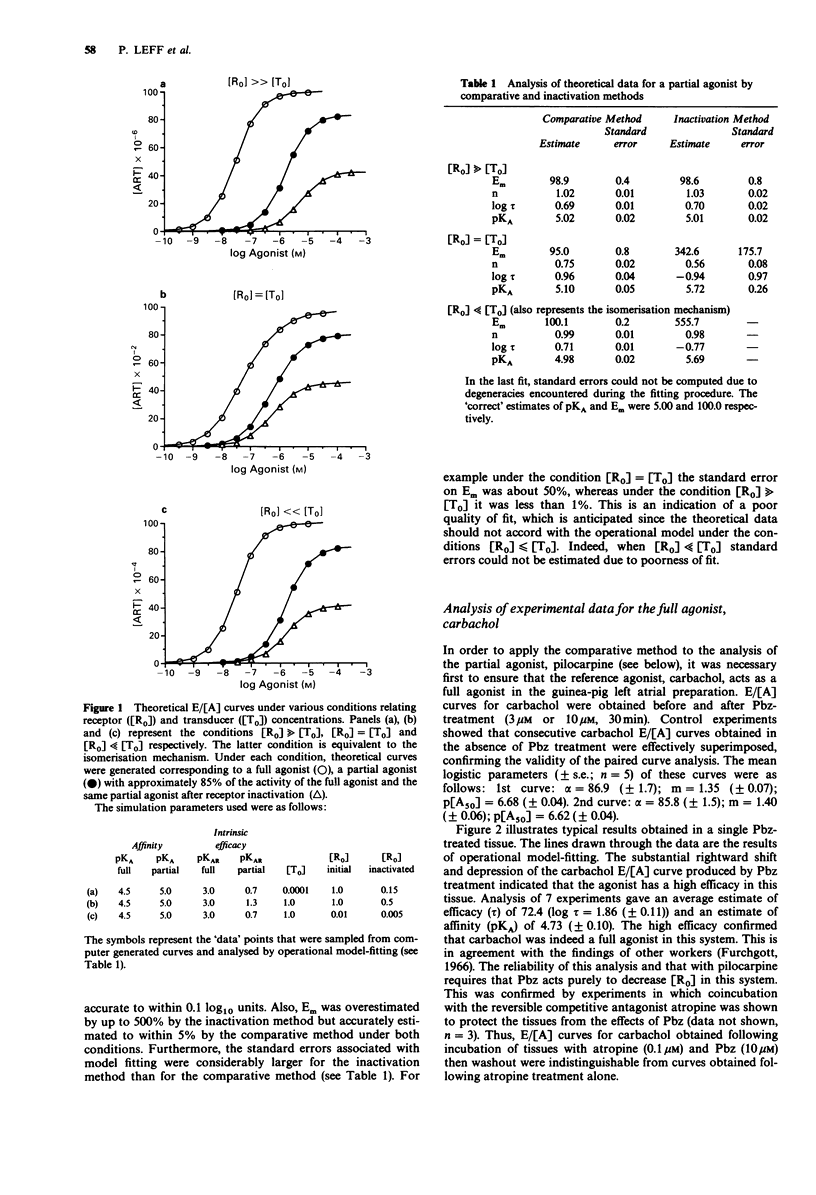
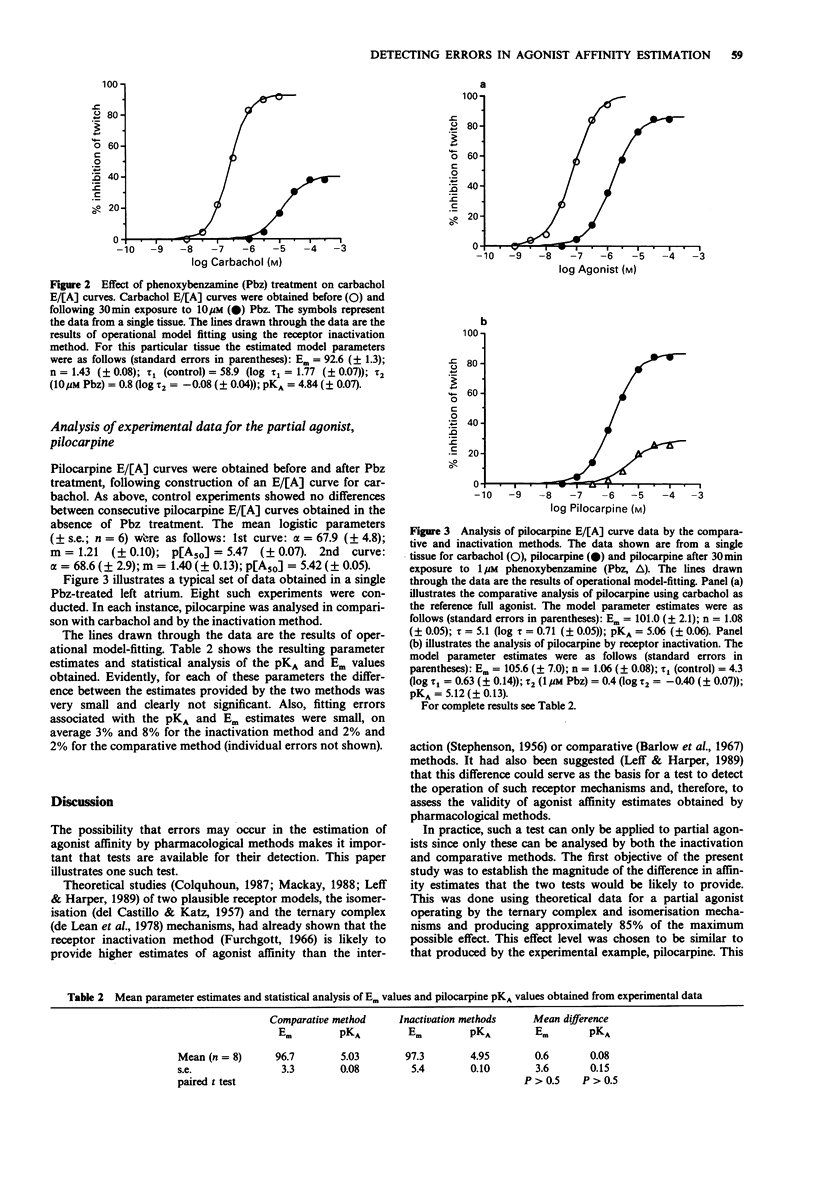
1. Recent theoretical studies have questioned the pharmacological estimation of agonist affinity. They showed that when receptor isomerisation or ternary complex mechanisms operate, the receptor inactivation method can substantially overestimate affinity, whereas methods for partial agonist analysis are more accurate. We previously suggested that the operation of such mechanisms and therefore the presence of errors could be detected by analysing the same partial agonist by the receptor inactivation and comparative methods. This paper describes the practical application of this test. 2. The ternary complex mechanism was simulated for a partial agonist under various conditions relating receptor (R) and transducer (T) concentrations, one of which also corresponds to the receptor isomerisation mechanism. The theoretical data so generated were then analysed by the inactivation and comparative methods to quantify the magnitude of error of affinity estimation that could occur. 3. This analysis showed that for a partial agonist with approximately 85% of the activity of a full agonist, the inactivation method could produce an affinity (pKA) estimate up to 0.7 log10 units higher than that produced by the comparative method. This difference would occur when the total receptor concentration ([R0]) is less than or equal to the total transducer concentration ([T0]). It also showed that the overestimation of affinity by the inactivation method was accompanied by drastic overestimation of Em, the maximal effect parameter. 4. The test was then exemplified using the muscarinic receptor system in the guinea-pig isolated left atrial preparation, where there is evidence that a ternary complex mechanism operates. The test agonist was pilocarpine, which produced on average 83% of the activity of the full agonist, carbachol.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. B., Scott N. C., Stephenson R. P. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967 Sep;31(1):188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P., Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985 Feb;84(2):561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- De Lean A., Stadel J. M., Lefkowitz R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980 Aug 10;255(15):7108–7117. [PubMed] [Google Scholar]
- Eglen R. M., Huff M. M., Montgomery W. W., Whiting R. L. Differential effects of pertussis toxin and lithium on muscarinic responses in the atria and ileum: evidence for receptor heterogeneity. Br J Pharmacol. 1987 May;91(1):6–8. doi: 10.1111/j.1476-5381.1987.tb08976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. P. Challenges for receptor theory as a tool for drug and drug receptor classification. Trends Pharmacol Sci. 1989 Jan;10(1):18–22. doi: 10.1016/0165-6147(89)90102-8. [DOI] [PubMed] [Google Scholar]
- Leff P., Dougall I. G., Harper D. H., Dainty I. A. Errors in agonist affinity estimation: do they and should they occur in isolated tissue experiments? Trends Pharmacol Sci. 1990 Feb;11(2):64–67. doi: 10.1016/0165-6147(90)90319-4. [DOI] [PubMed] [Google Scholar]
- Leff P., Harper D. Do pharmacological methods for the quantification of agonists work when the ternary complex mechanism operates? J Theor Biol. 1989 Oct 9;140(3):381–397. doi: 10.1016/s0022-5193(89)80094-3. [DOI] [PubMed] [Google Scholar]
- Leff P., Prentice D. J., Giles H., Martin G. R., Wood J. Estimation of agonist affinity and efficacy by direct, operational model-fitting. J Pharmacol Methods. 1990 May;23(3):225–237. doi: 10.1016/0160-5402(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Mackay D. Continuous variation of agonist affinity constants. Trends Pharmacol Sci. 1988 May;9(5):156–157. doi: 10.1016/0165-6147(88)90026-0. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud D. R. On the measurement of the affinity of partial agonists for receptors. J Pharmacol Exp Ther. 1969 Nov;170(1):117–122. [PubMed] [Google Scholar]


