Abstract
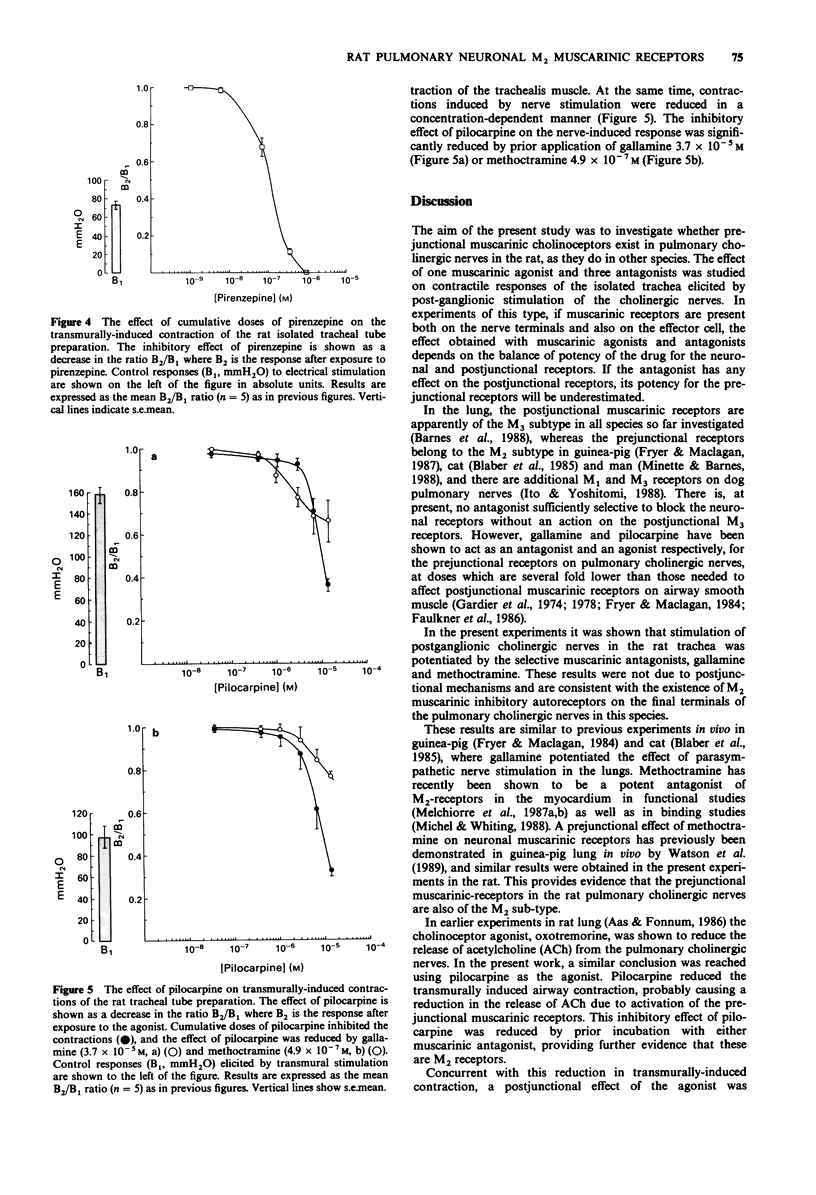
1. The effects of muscarinic antagonists considered to be selective for M1 receptors (pirenzepine) and for M2 receptors (gallamine and methoctramine) were used to investigate the existence of prejunctional muscarinic receptors on cholinergic nerves in the rat lung. The tracheal tube preparation was used in vitro, and contraction of the trachealis muscle was induced by electrical field stimulation (EFS) and by application of an exogenous muscarinic agonist (pilocarpine), and measured as an increase in intraluminal pressure in the tube. 2. The muscarinic antagonists, gallamine and methoctramine, enhanced the contractions induced by nerve stimulation, while contractions elicited by exogenous application of pilocarpine were inhibited by the antagonists. 3. In contrast, pirenzepine blocked contractions induced by both EFS and pilocarpine in a dose-dependent manner (EC50 0.1 microM) due to blockade of the postjunctional muscarinic receptors on airway smooth muscle. Potentiation of the response to EFS was never seen with this antagonist. 4. The muscarinic agonist, pilocarpine, caused a slow maintained increase in tone of the tracheal tube and at the same time reduced the contractions induced by EFS. This inhibitory effect was blocked by gallamine and methoctramine. 5. The results suggest that prejunctional inhibitory muscarinic receptors may be localised on the parasympathetic cholinergic nerve terminals innervating tracheal smooth muscle in the rat. This confirms previous findings obtained by measuring transmitter release in this species. The present results suggest that these receptors are of the M2 subtype. Blockade of these autoreceptors with gallamine or methoctramine would increase the output of acetylcholine (ACh) and thereby enhance the nerve-induced contraction of tracheal smooth muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas P., Fonnum F. Presynaptic inhibition of acetylcholine release. Acta Physiol Scand. 1986 Jul;127(3):335–342. doi: 10.1111/j.1748-1716.1986.tb07913.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Minette P., Maclagan J. Muscarinic receptor subtypes in airways. Trends Pharmacol Sci. 1988 Nov;9(11):412–416. doi: 10.1016/0165-6147(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Blaber L. C., Fryer A. D., Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol. 1985 Nov;86(3):723–728. doi: 10.1111/j.1476-5381.1985.tb08951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Farmer J. B., Coleman R. A. A new preparation of the isolated intact trachea of the guinea-pig. J Pharm Pharmacol. 1970 Jan;22(1):46–50. doi: 10.1111/j.2042-7158.1970.tb08383.x. [DOI] [PubMed] [Google Scholar]
- Faulkner D., Fryer A. D., Maclagan J. Postganglionic muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1986 May;88(1):181–187. doi: 10.1111/j.1476-5381.1986.tb09485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984 Dec;83(4):973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Pancuronium and gallamine are antagonists for pre- and post-junctional muscarinic receptors in the guinea-pig lung. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):367–371. doi: 10.1007/BF00165549. [DOI] [PubMed] [Google Scholar]
- Gardier R. W., Ganansia M. F., Delaunois A. L., Hamelberg W. Enhancement of ganglionic muscarinic activity by gallamine. Anesthesiology. 1974 May;40(5):494–497. doi: 10.1097/00000542-197405000-00016. [DOI] [PubMed] [Google Scholar]
- Gardier R. W., Tsevdos E. J., Jackson D. B. The effect of pancuronium and gallamine on muscarinic transmission in the superior cervical ganglion. J Pharmacol Exp Ther. 1978 Jan;204(1):46–53. [PubMed] [Google Scholar]
- Ito Y., Yoshitomi T. Autoregulation of acetylcholine release from vagus nerve terminals through activation of muscarinic receptors in the dog trachea. Br J Pharmacol. 1988 Mar;93(3):636–646. doi: 10.1111/j.1476-5381.1988.tb10321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorre C., Angeli P., Lambrecht G., Mutschler E., Picchio M. T., Wess J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur J Pharmacol. 1987 Dec 1;144(2):117–124. doi: 10.1016/0014-2999(87)90509-7. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Cassinelli A., Quaglia W. Differential blockade of muscarinic receptor subtypes by polymethylene tetraamines. Novel class of selective antagonists of cardiac M-2 muscarinic receptors. J Med Chem. 1987 Jan;30(1):201–204. doi: 10.1021/jm00384a034. [DOI] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Methoctramine, a polymethylene tetraamine, differentiates three subtypes of muscarinic receptor in direct binding studies. Eur J Pharmacol. 1988 Jan 5;145(1):61–66. doi: 10.1016/0014-2999(88)90349-4. [DOI] [PubMed] [Google Scholar]
- Minette P. A., Barnes P. J. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol (1985) 1988 Jun;64(6):2532–2537. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- Walters E. H., O'Byrne P. M., Fabbri L. M., Graf P. D., Holtzman M. J., Nadel J. A. Control of neurotransmission by prostaglandins in canine trachealis smooth muscle. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):129–134. doi: 10.1152/jappl.1984.57.1.129. [DOI] [PubMed] [Google Scholar]


