Abstract
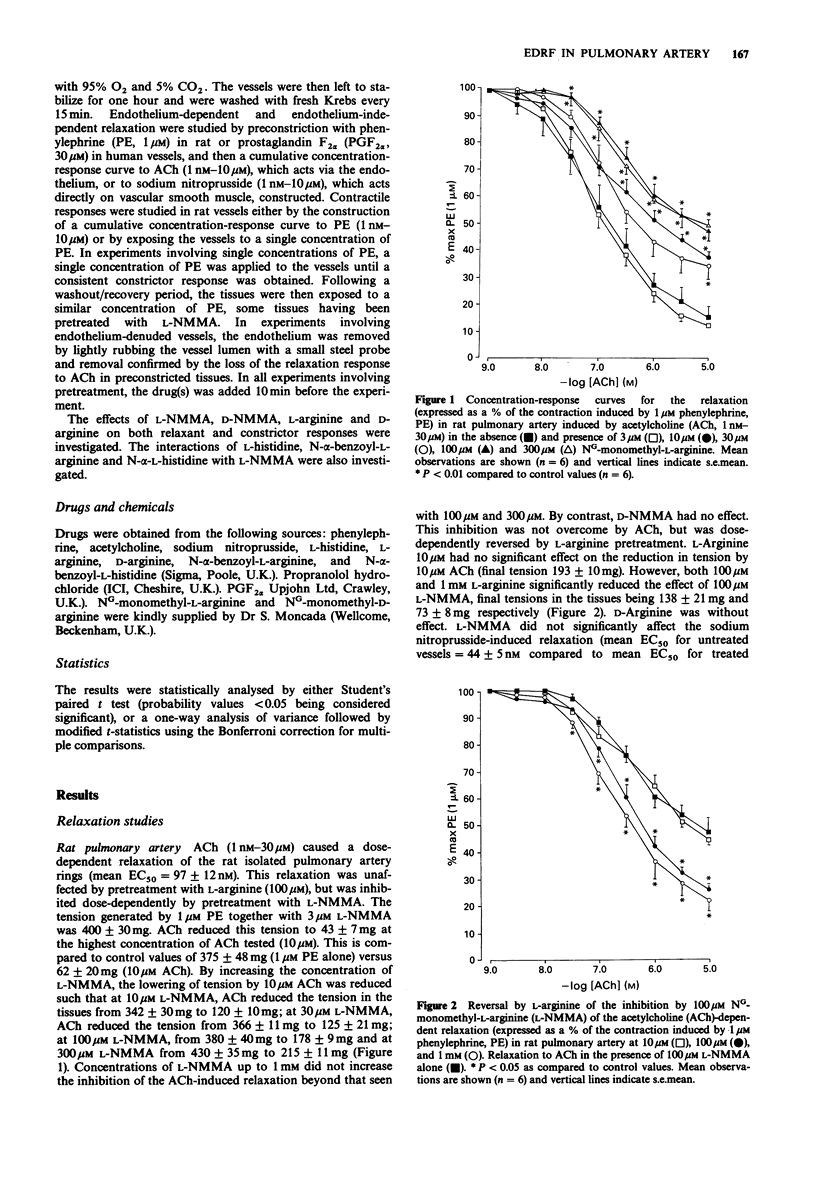
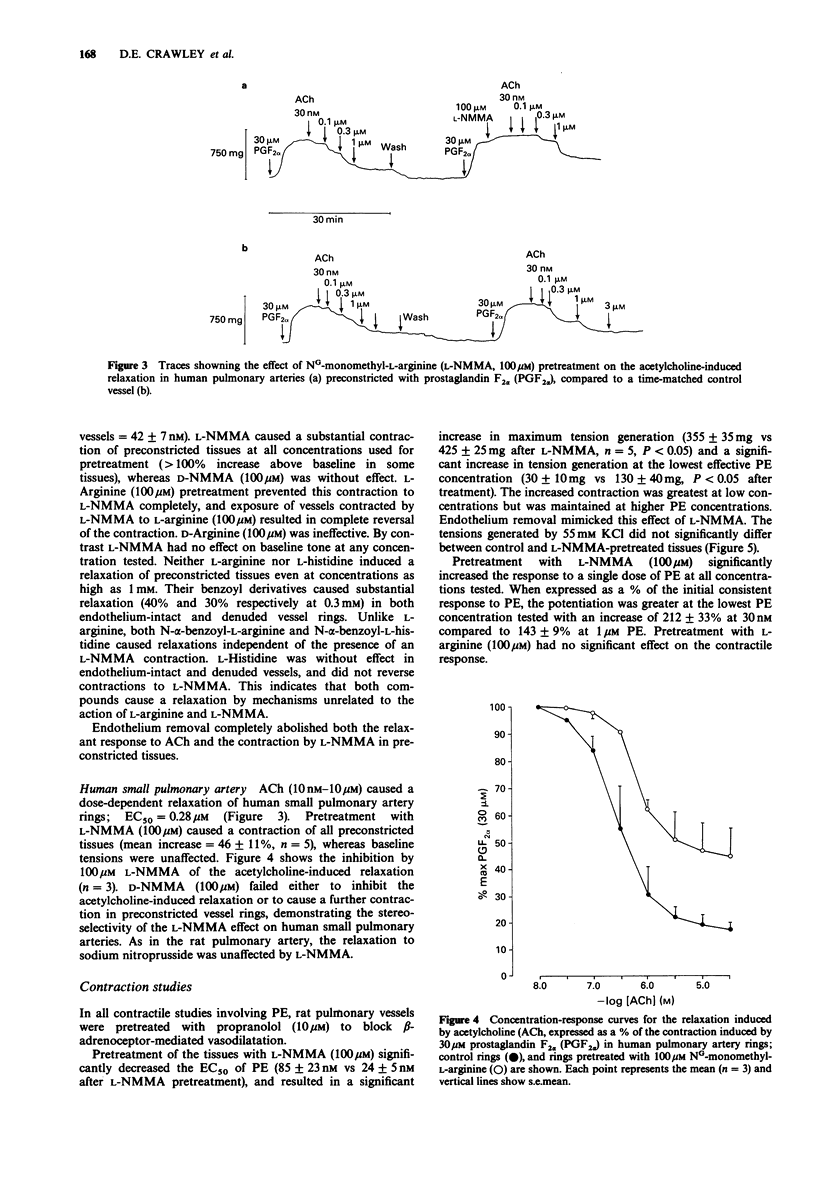
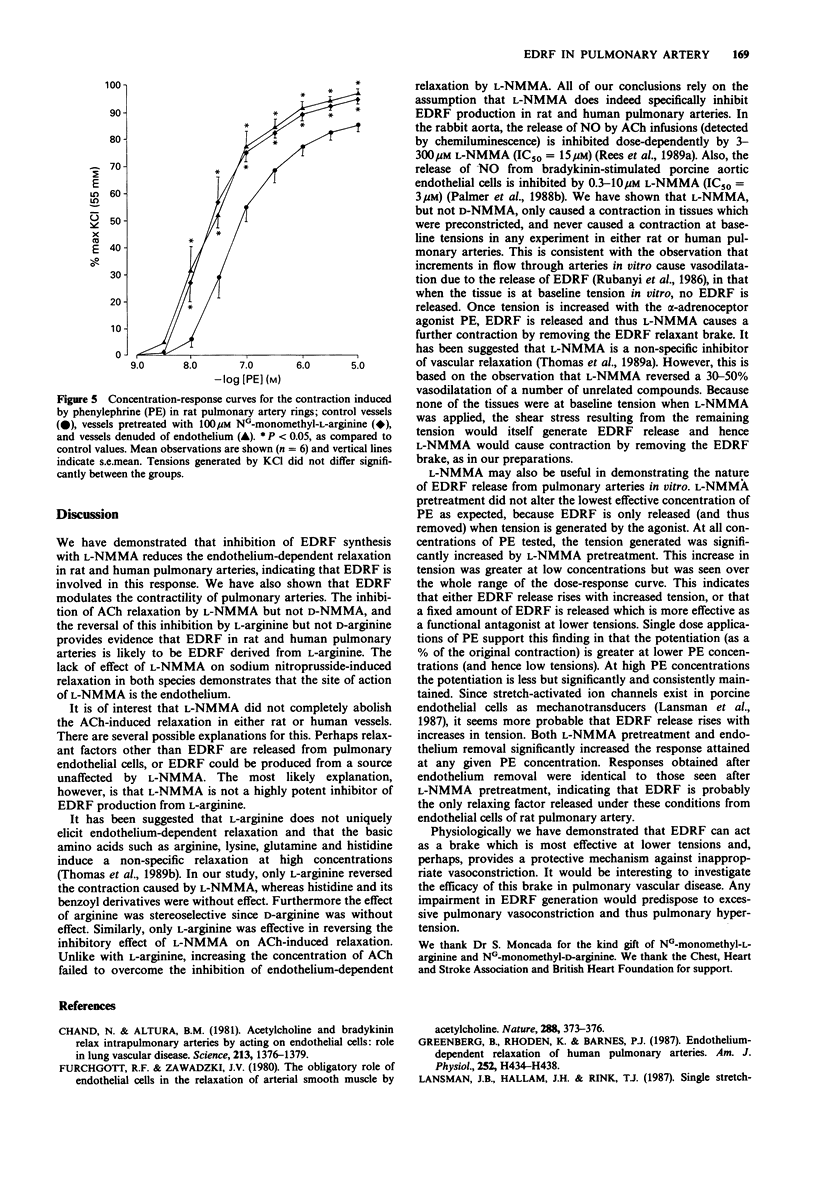
1. The inhibitory role of endothelium-derived relaxing factor was studied in both rat and human pulmonary arteries in vitro by inhibiting its synthesis with the L-arginine analogue NG-monomethyl-L-arginine (L-NMMA). 2. In rat pulmonary arteries, L-NMMA pretreatment (10-300 microM) dose-dependently inhibited acetylcholine-induced relaxation (which is endothelium-dependent). NG-monomethyl-D-arginine (D-NMMA, 100 microM) was without effect. L-Arginine, but not D-arginine, dose-dependently reversed this inhibition. L-NMMA had no effect on relaxation induced by sodium nitroprusside. 3. In human small pulmonary arteries L-NMMA (100 microM) pretreatment similarly inhibited the acetylcholine-induced relaxation but had no effect on the sodium nitroprusside-induced relaxation. 4. In both rat and human pulmonary arteries, L-NMMA, but not D-NMMA, always caused contraction of preconstricted tissues whereas it had no effect on baseline tone. In the rat this contraction was completely prevented by prior treatment with L-arginine. 5. L-NMMA (100 microM) pretreatment mimicked the effect of endothelium removal on phenylephrine-induced vasoconstriction, both resulting in an increase in tension development at each concentration of phenylephrine. This enhancement was greatest at low concentrations of phenylephrine but was still present even at the highest concentrations. Pretreatment with L-NMMA (100 microM) also significantly increased the responses to single doses of phenylephrine. 6. These results suggest that endothelium-derived relaxing factor from endothelial cells both mediates the relaxation response to acetylcholine and also acts as a physiological brake against vasoconstriction in pulmonary vessels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Greenberg B., Rhoden K., Barnes P. J. Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol. 1987 Feb;252(2 Pt 2):H434–H438. doi: 10.1152/ajpheart.1987.252.2.H434. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Cole E. A., Ramwell P. W. NG-monomethyl L-arginine is a non-specific inhibitor of vascular relaxation. Eur J Pharmacol. 1989 Oct 24;170(1-2):123–124. doi: 10.1016/0014-2999(89)90148-9. [DOI] [PubMed] [Google Scholar]
- Thomas G., Hecker M., Ramwell P. W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Jan 16;158(1):177–180. doi: 10.1016/s0006-291x(89)80194-9. [DOI] [PubMed] [Google Scholar]
- Thomas G., Ramwell P. W. Vasodilatory properties of mono-L-arginine-containing compounds. Biochem Biophys Res Commun. 1988 Jul 15;154(1):332–338. doi: 10.1016/0006-291x(88)90689-4. [DOI] [PubMed] [Google Scholar]


