Abstract
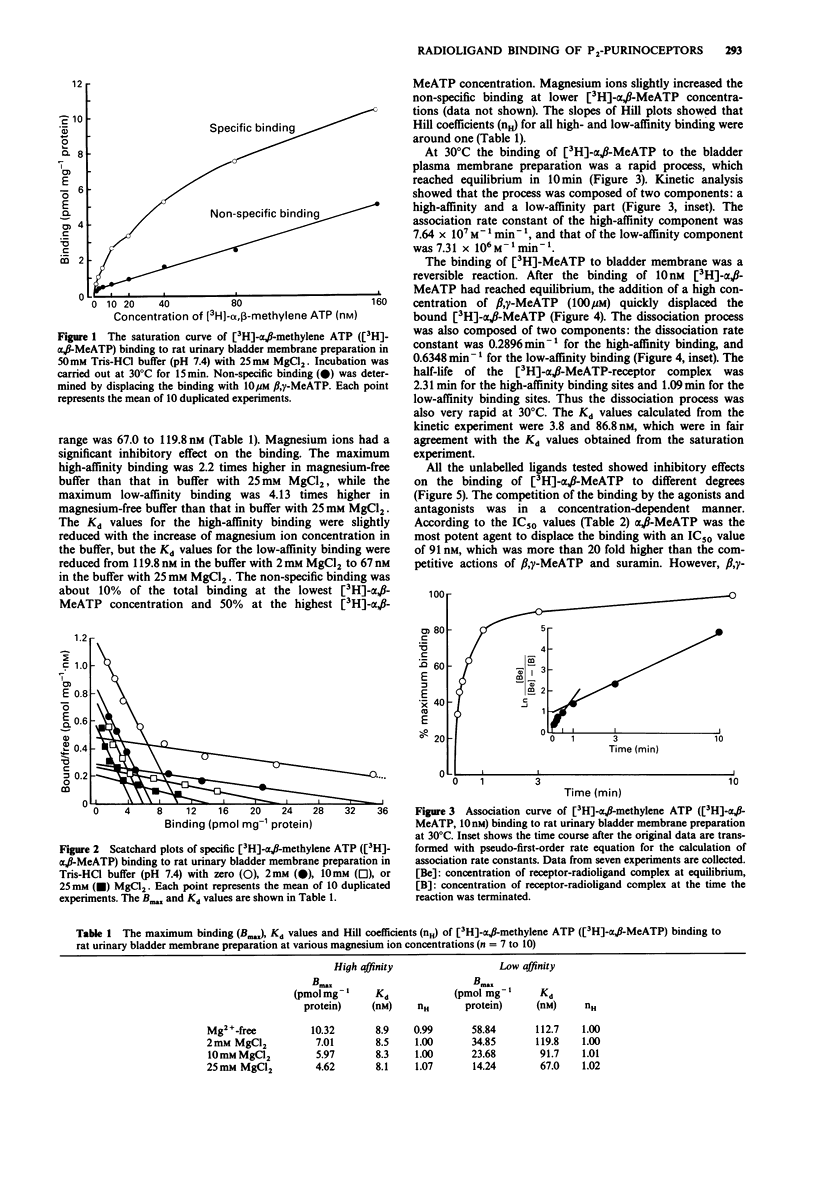
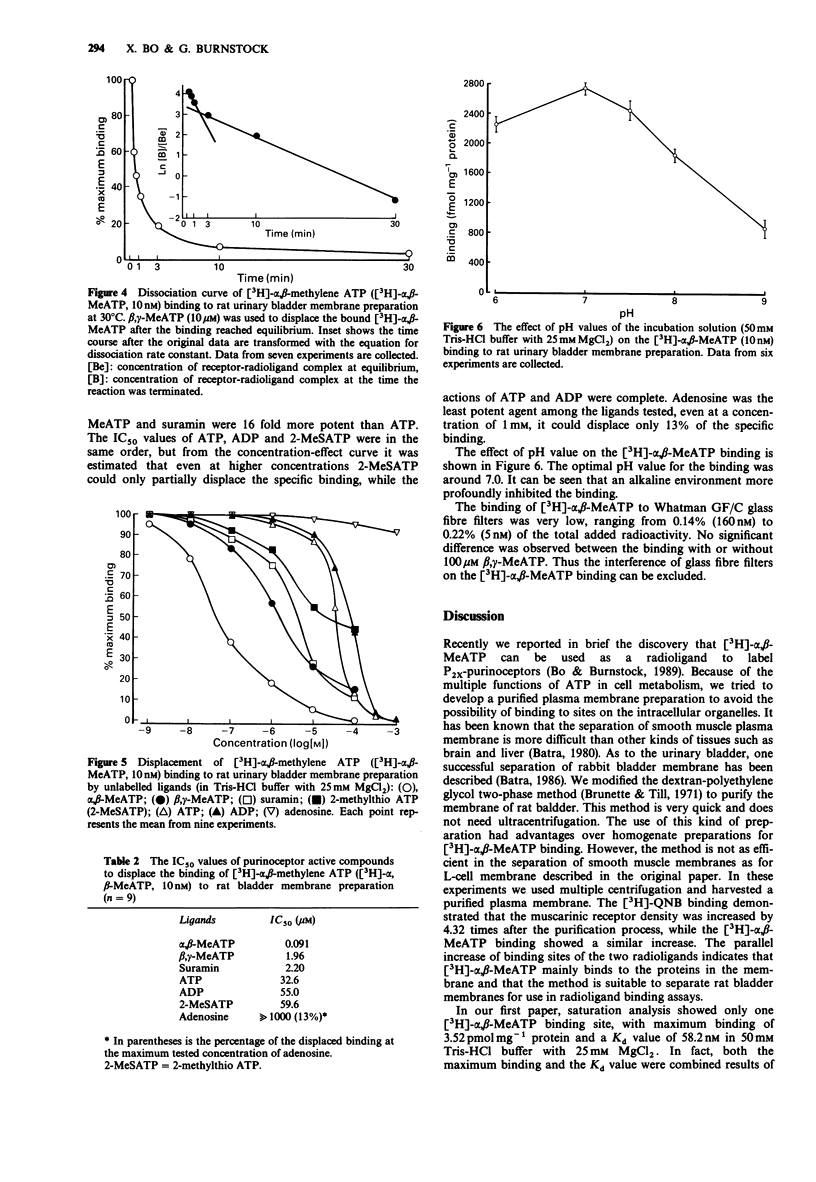
1. The characteristics of [3H]-alpha, beta-methylene adenosine 5'triphosphate ([3H]-alpha, beta-MeATP) binding to membrane preparations of rat urinary bladder detrusor were studied. 2. The rat bladder membrane preparation was obtained by multiple centrifugation. [3H]-quinuclidinyl benzilate [( 3H]-QNB) binding to this preparation demonstrated that the muscarinic receptor density was 4.32 times higher than that in the homogenate. [3H]-alpha, beta-MeATP binding was increased 3.88 times. 3. Saturation analysis revealed that the rat bladder membrane contained a high density of [3H]-alpha, beta-MeATP binding sites, which could be divided into a high-affinity component (Kd = 8.1-8.9 nM) and a low-affinity component (Kd = 67.0-119.8 nM). 4. Magnesium ions inhibited the maximum binding in a concentration-dependent manner. The maximum high-affinity binding was reduced from 10.32 pmol mg-1 protein in magnesium-free buffer to 4.62 pmol mg-1 protein with 25 mM MgCl2, while the maximum low-affinity binding was reduced from 58.84 pmol mg-1 protein to 14.24 pmol mg-1 protein. Kd values were not greatly affected. 5. The binding was a rapid reversible process. The association rate constants were 7.64 x 10(7) M-1 min-1 for high-affinity binding, and 7.31 x 10(6) M-1 min-1 for low-affinity binding. The dissociation rate constants were 0.2896 min-1 for high-affinity binding, and 0.6348 min-1 for the low-affinity binding. 6. Displacement experiments with unlabelled purinoceptor ligands confirmed that [3H]-alpha, beta-MeATP mainly binds to P2X-purinoceptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo C. G., Contreras E. Possible involvement of adenine nucleotides in the neurotransmission of the mouse urinary bladder. Comp Biochem Physiol C. 1985;82(2):357–361. doi: 10.1016/0742-8413(85)90176-8. [DOI] [PubMed] [Google Scholar]
- Batra S. Use of 3H-QNB in the isolation of plasma membrane from smooth muscle of the urinary bladder: effect of oxalate on calcium uptake by the membrane fractions. Experientia. 1986 Jun 15;42(6):608–611. doi: 10.1007/BF01955556. [DOI] [PubMed] [Google Scholar]
- Bo X. N., Burnstock G. [3H]-alpha, beta-methylene ATP, a radioligand labelling P2-purinoceptors. J Auton Nerv Syst. 1989 Oct;28(1):85–88. doi: 10.1016/0165-1838(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Morris A. J., Harden T. K. Guanine nucleotide-sensitive interaction of a radiolabeled agonist with a phospholipase C-linked P2y-purinergic receptor. J Biol Chem. 1989 Apr 15;264(11):6202–6206. [PubMed] [Google Scholar]
- Cooper D. M., Yeung S. M., Perez-Reyes E., Fossom L. H. A central role for magnesium in the regulation of inhibitory adenosine receptors. Adv Exp Med Biol. 1984;175:17–30. doi: 10.1007/978-1-4684-4805-4_2. [DOI] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Contribution of adrenergic and "purinergic" neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther. 1978 Nov;207(2):431–445. [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Cooper M. J., Gavish M., Snyder S. H. Guanine nucleotide and cation regulation of the binding of [3H]cyclohexyladenosine and [3H]diethylphenylxanthine to adenosine A1 receptors in brain membranes. Mol Pharmacol. 1982 Mar;21(2):329–335. [PubMed] [Google Scholar]
- Hourani S. M., Welford L. A., Cusack N. J. L-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol. 1985 Jan 22;108(2):197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Chapple C., Burnstock G. Isolated human bladder: evidence for an adenine dinucleotide acting on P2X-purinoceptors and for purinergic transmission. Eur J Pharmacol. 1989 Dec 12;174(1):115–118. doi: 10.1016/0014-2999(89)90881-9. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S., Sjögren C., Andersson K. E. Direct effects of adenosine and adenine nucleotides on isolated human urinary bladder and their influence on electrically induced contractions. J Urol. 1983 Aug;130(2):392–398. doi: 10.1016/s0022-5347(17)51175-1. [DOI] [PubMed] [Google Scholar]
- Hüttemann E., Ukena D., Lenschow V., Schwabe U. Ra adenosine receptors in human platelets. Characterization by 5'-N-ethylcarboxamido[3H]adenosine binding in relation to adenylate cyclase activity. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):226–233. doi: 10.1007/BF00495948. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986 Dec 1;240(2):367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Characterization of purinoceptors present on human liver plasma membranes. FEBS Lett. 1989 May 8;248(1-2):137–140. doi: 10.1016/0014-5793(89)80448-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin R. M., Jacoby R., Wein A. J. High-affinity, divalent ion-specific binding of 3H-ATP to homogenate derived from rabbit urinary bladder. Comparison with divalent-ion ATPase activity. Mol Pharmacol. 1983 Jan;23(1):1–7. [PubMed] [Google Scholar]
- Moss H. E., Burnstock G. A comparative study of electrical field stimulation of the guinea-pig, ferret and marmoset urinary bladder. Eur J Pharmacol. 1985 Aug 27;114(3):311–316. doi: 10.1016/0014-2999(85)90375-9. [DOI] [PubMed] [Google Scholar]
- Theobald R. J., Jr The effect of arylazido aminopropionyl ATP on atropine resistant contractions of the cat urinary bladder. Life Sci. 1983 May 23;32(21):2479–2484. doi: 10.1016/0024-3205(83)90374-0. [DOI] [PubMed] [Google Scholar]


