Abstract
1 The pharmacological properties of the benzodiazepine receptor ligand, FG 8205 (7-chloro-5,6-dihydro-5-methyl-6-oxo-3-(5-isopropyl-1,2,4-oxadiazol-3-yl)-4H- imidazol[1,5a][1,4]benzodiazepine) have been examined.
2 FG 8205 potently displaced [3H]-flumazenil binding in rat cortical membranes with a K1 of 3.3 nM, but was inactive at 13 neurotransmitter recognition sites.
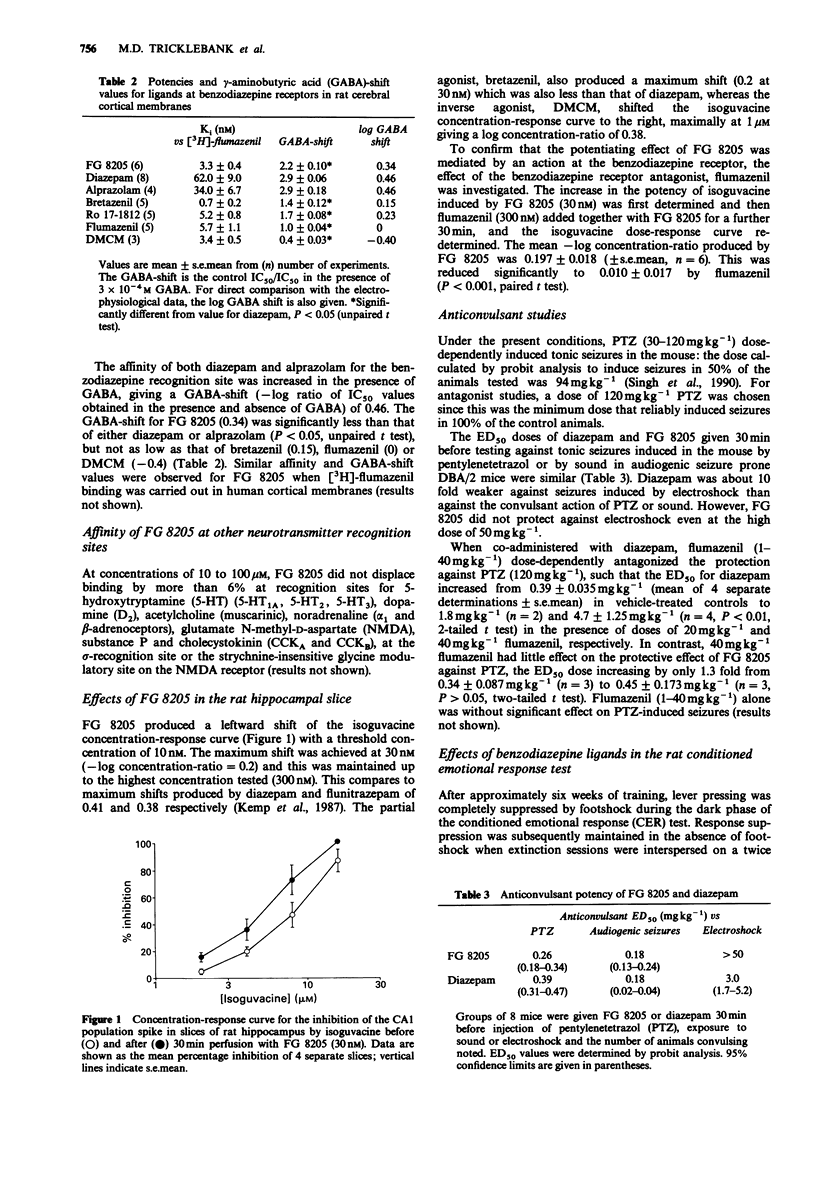
3 Consistent with a partial agonist profile, the affinity of FG 8205 for the benzodiazepine recognition site was increased in the presence of γ-aminobutyric acid (GABA, 300μM) by a degree (—log [IC50 in the presence of GABA/IC50 alone] = 0.34) significantly less than found for diazepam (0.46). FG 8205 also potentiated the inhibitory potency of the GABAA-receptor agonist, isoguvacine, on the hippocampal CA1 population spike and, again, the maximum shift (—log dose-ratio = 0.2) was significantly less than that seen with diazepam (0.4).
4 In anticonvulsant studies, the ED50 doses of FG 8205 and diazepam needed to antagonize seizures induced by pentylenetetrazol (PTZ) or by sound in audiogenic seizure prone mice were similar with values of 0.2–0.3 mgkg-1, i.p. However, even high doses of FG 8205 (50 mgkg-1) did not protect against seizures induced by electroshock.
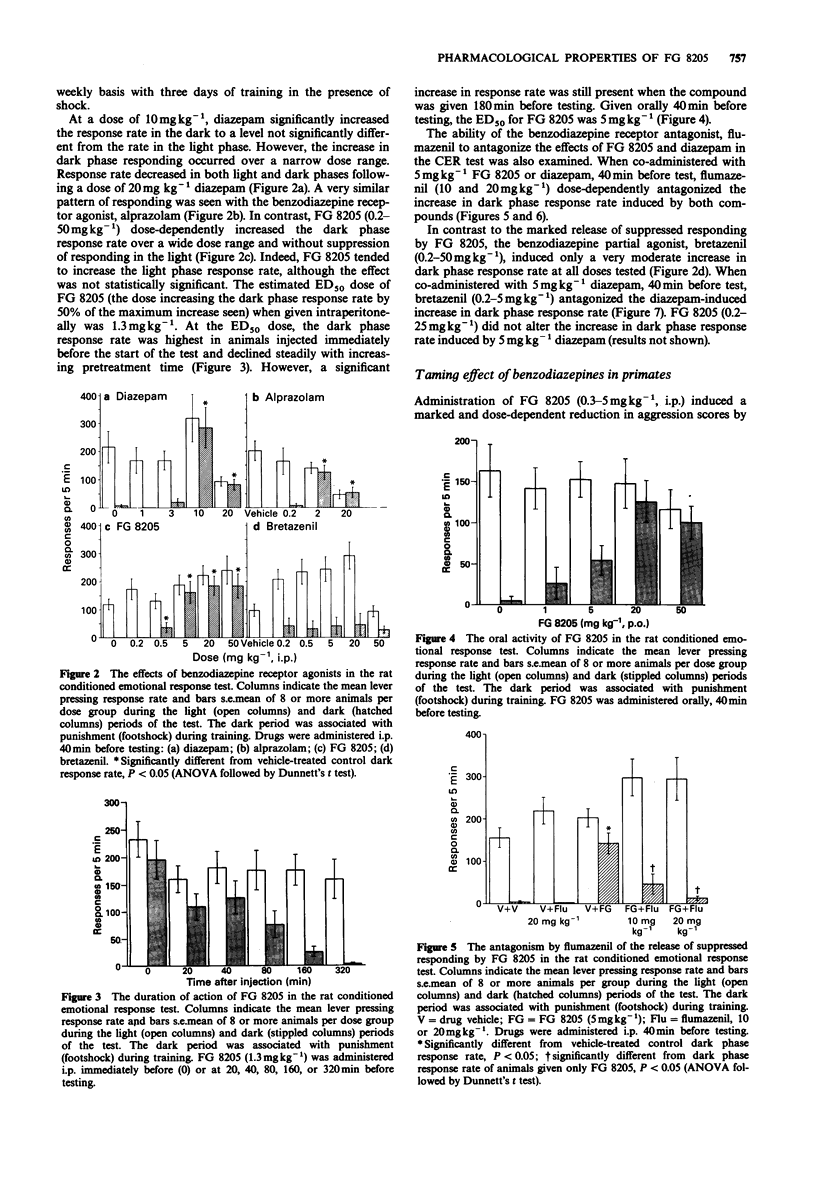
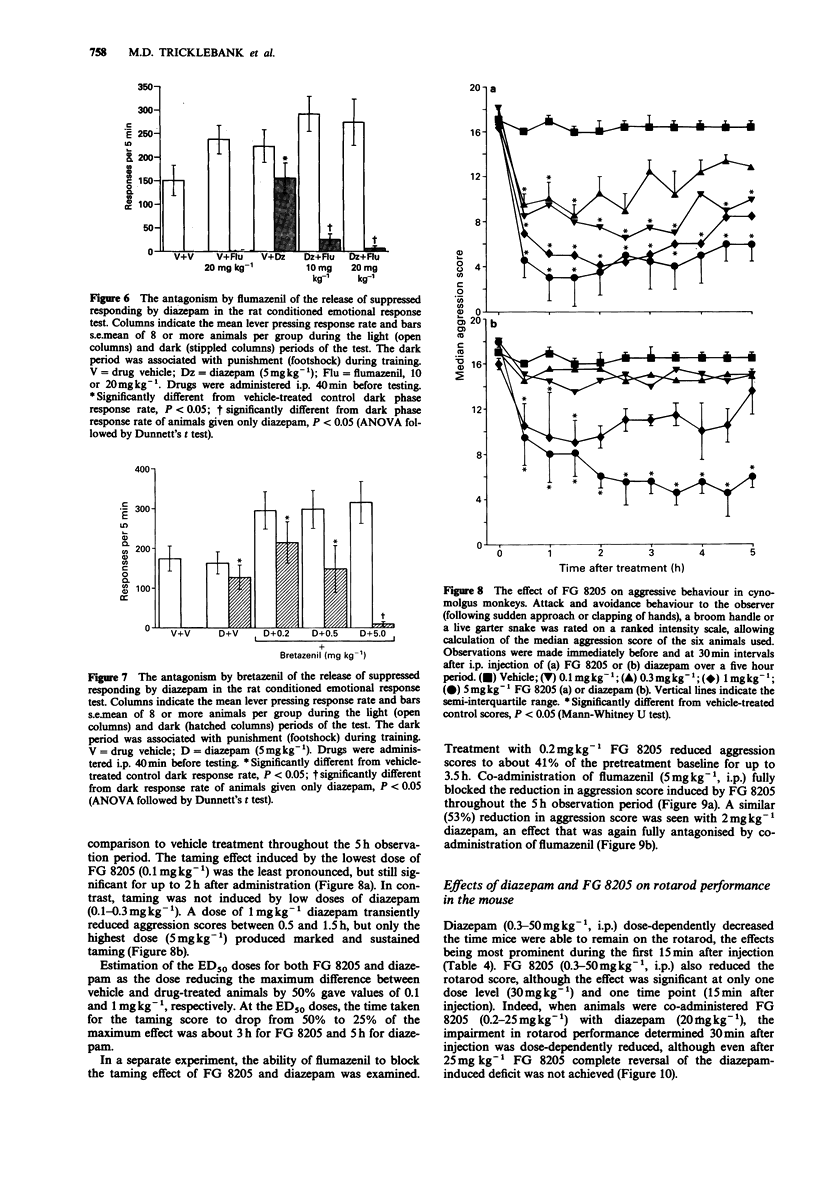
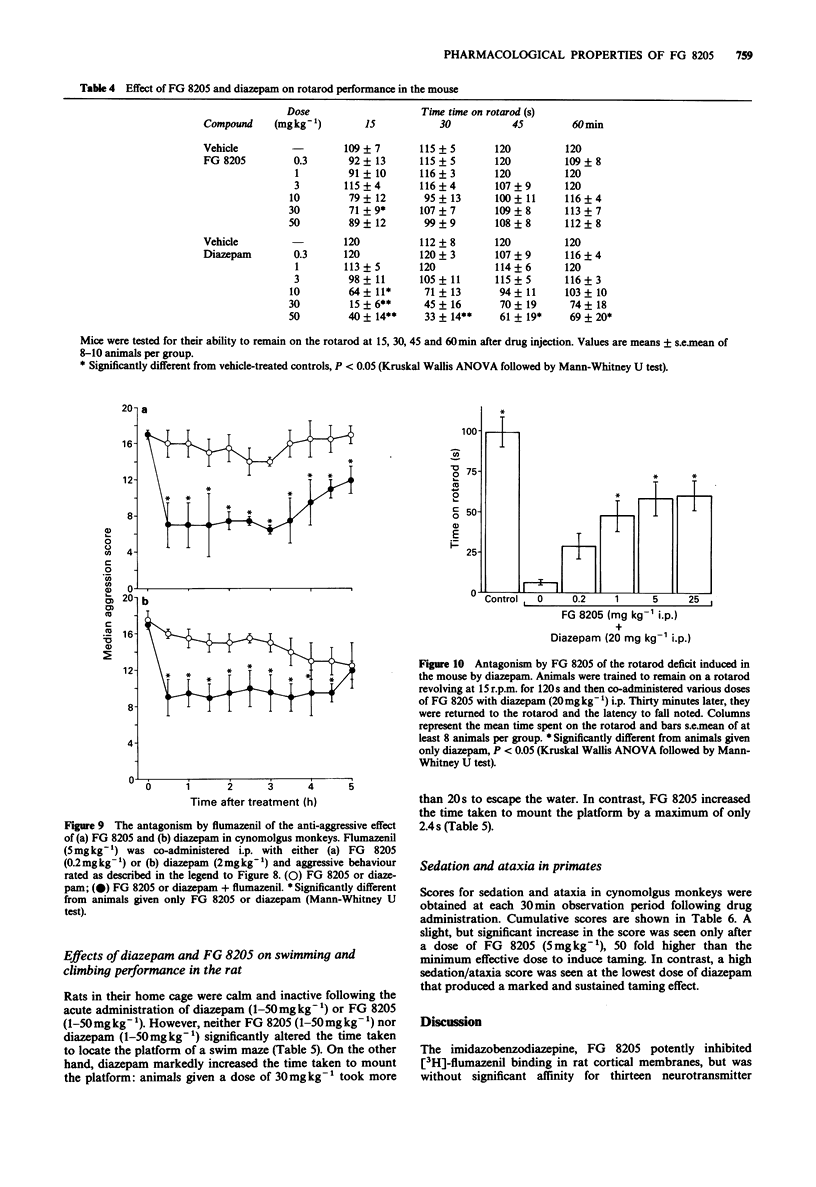
5 FG 8205 released responding suppressed by footshock in a rat operant conditioned emotional response task over the dose range 0.5–50 mgkg-1 (i.p.). Similar doses of FG 8205 had a marked taming effect in cynomolgus monkeys. However, measures of sedation and ataxia (as measured by rotarod in the mouse, climbing behaviour in the rat, and by scoring arousal and co-ordination in primates) were slight and only transiently affected by FG 8205, and FG 8205 significantly antagonized the rotarod performance deficit induced by diazepam in the mouse.
6 While the potentiation by FG 8205 of the response to isoguvacine in the rat hippocampal slice and the anxiolytic-like effects of the compound in both rats and primates were reversed by the benzodiazepine receptor antagonist, flumazenil, high doses of the antagonist were able only marginally to block the protective effects of FG 8205 against seizures induced by PTZ in the mouse.
7 Thus, FG 8205 does not show the marked motor impairment characteristic of full agonists at the benzodiazepine receptor, consistent with its partial agonist profile in in vitro assay systems. Nevertheless, the compound has sufficient intrinsic activity to maintain high efficacy in anticonvulsant and anxiolytic tests.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braestrup C., Honoré T., Nielsen M., Petersen E. N., Jensen L. H. Ligands for benzodiazepine receptors with positive and negative efficacy. Biochem Pharmacol. 1984 Mar 15;33(6):859–862. doi: 10.1016/0006-2952(84)90438-6. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Schmiechen R., Neef G., Nielsen M., Petersen E. N. Interaction of convulsive ligands with benzodiazepine receptors. Science. 1982 Jun 11;216(4551):1241–1243. doi: 10.1126/science.6281892. [DOI] [PubMed] [Google Scholar]
- Ehlert F. J., Ragan P., Chen A., Roeske W. R., Yamamura H. I. Modulation of benzodiazepine receptor binding: insight into pharmacological efficacy. Eur J Pharmacol. 1982 Feb 26;78(2):249–253. doi: 10.1016/0014-2999(82)90246-1. [DOI] [PubMed] [Google Scholar]
- Hunkeler W., Möhler H., Pieri L., Polc P., Bonetti E. P., Cumin R., Schaffner R., Haefely W. Selective antagonists of benzodiazepines. Nature. 1981 Apr 9;290(5806):514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Marshall G. R., Wong E. H., Woodruff G. N. The affinities, potencies and efficacies of some benzodiazepine-receptor agonists, antagonists and inverse-agonists at rat hippocampal GABAA-receptors. Br J Pharmacol. 1987 Jul;91(3):601–608. doi: 10.1111/j.1476-5381.1987.tb11253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Polc P., Bonetti E. P., Schaffner R., Haefely W. A three-state model of the benzodiazepine receptor explains the interactions between the benzodiazepine antagonist Ro 15-1788, benzodiazepine tranquilizers, beta-carbolines, and phenobarbitone. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(4):260–264. doi: 10.1007/BF00498510. [DOI] [PubMed] [Google Scholar]
- Potier M. C., Prado de Carvalho L., Venault P., Chapouthier G., Rossier J. Demonstration of the partial agonist profiles of Ro 16-6028 and Ro 17-1812 in mice in vivo. Eur J Pharmacol. 1988 Oct 26;156(1):169–172. doi: 10.1016/0014-2999(88)90161-6. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Lüddens H., Seeburg P. H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989 Sep 22;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- RANDALL L. O., HEISE G. A., SCHALLEK W., BAGDON R. E., BANZIGER R., BORIS A., MOE R. A., ABRAMS W. B. Pharmacological and clinical studies on Valium (T.M.) a new psychotherapeutic agent of the benzodiazepine class. Curr Ther Res Clin Exp. 1961 Sep;3:405–425. [PubMed] [Google Scholar]
- Singh L., Oles R. J., Tricklebank M. D. Modulation of seizure susceptibility in the mouse by the strychnine-insensitive glycine recognition site of the NMDA receptor/ion channel complex. Br J Pharmacol. 1990 Feb;99(2):285–288. doi: 10.1111/j.1476-5381.1990.tb14695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Iversen L. L. Modulation of [3H]diazepam binding in rat cortical membranes by GABAA agonists. J Neurochem. 1985 Apr;44(4):1162–1167. doi: 10.1111/j.1471-4159.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]


