Abstract
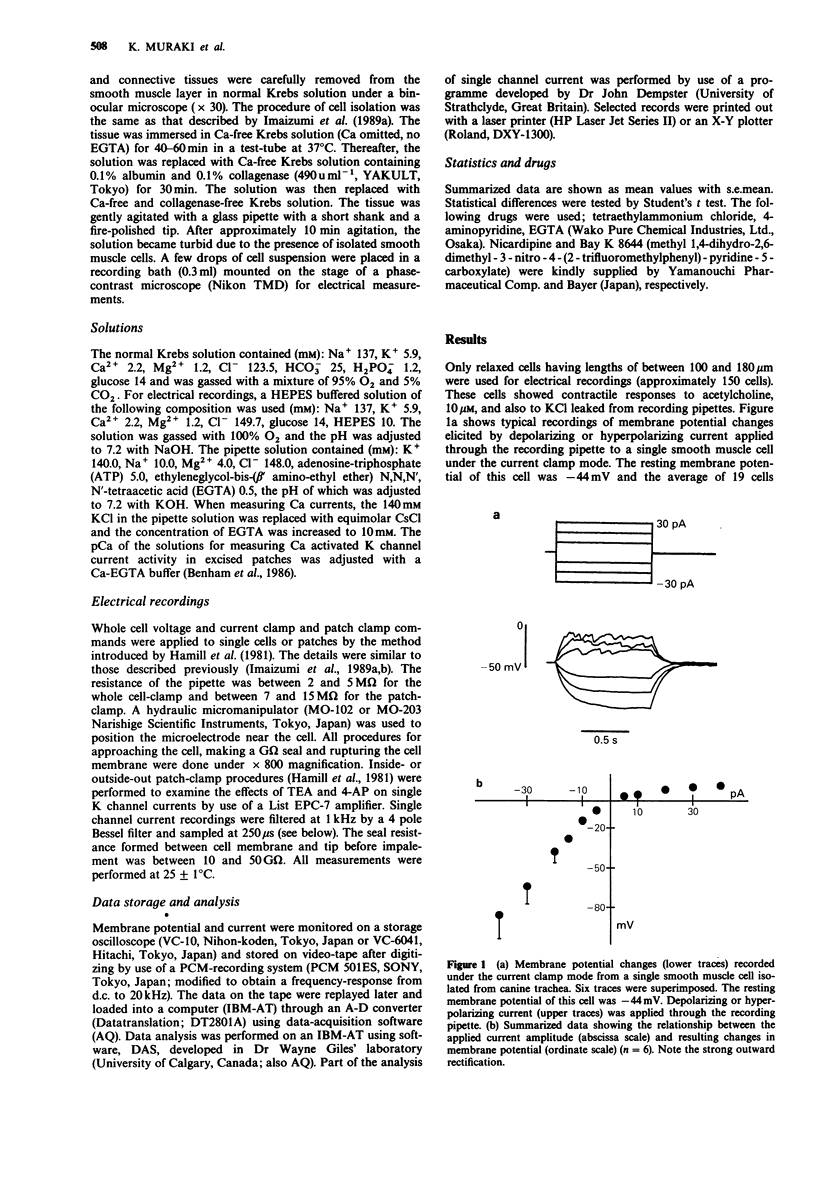
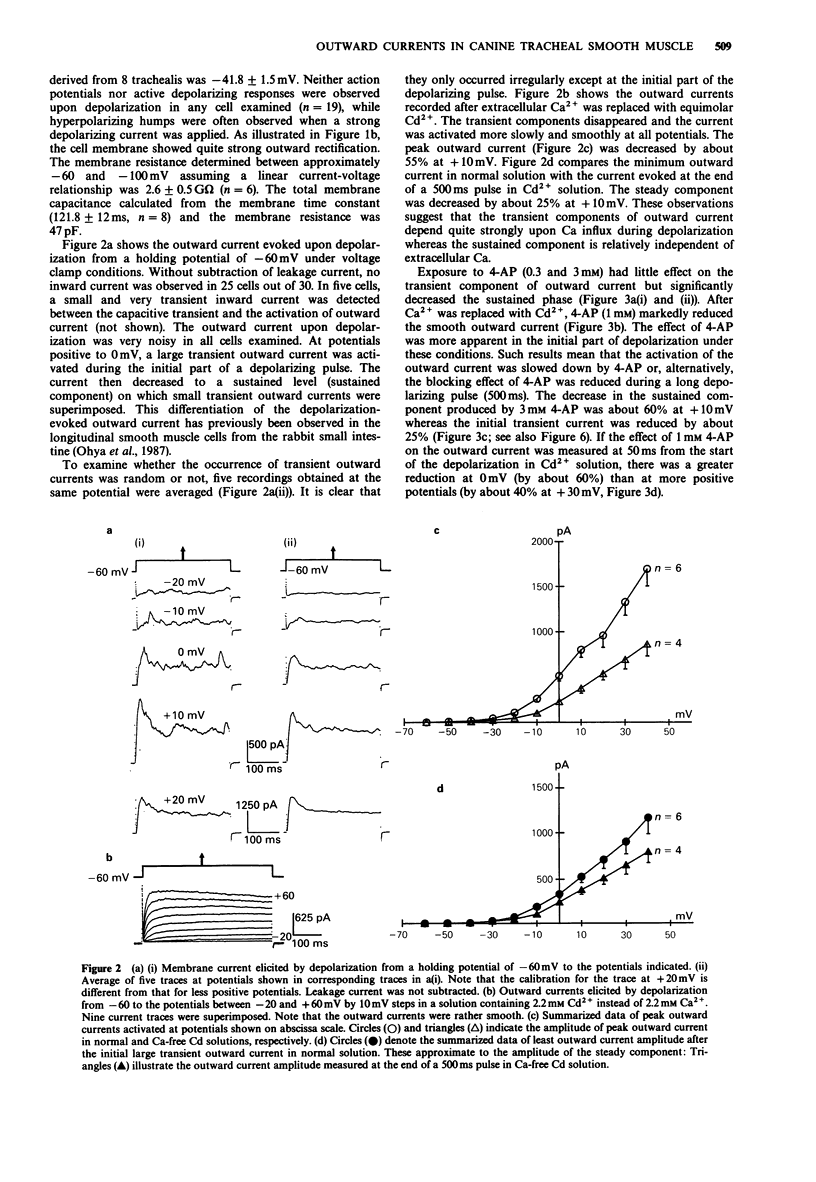
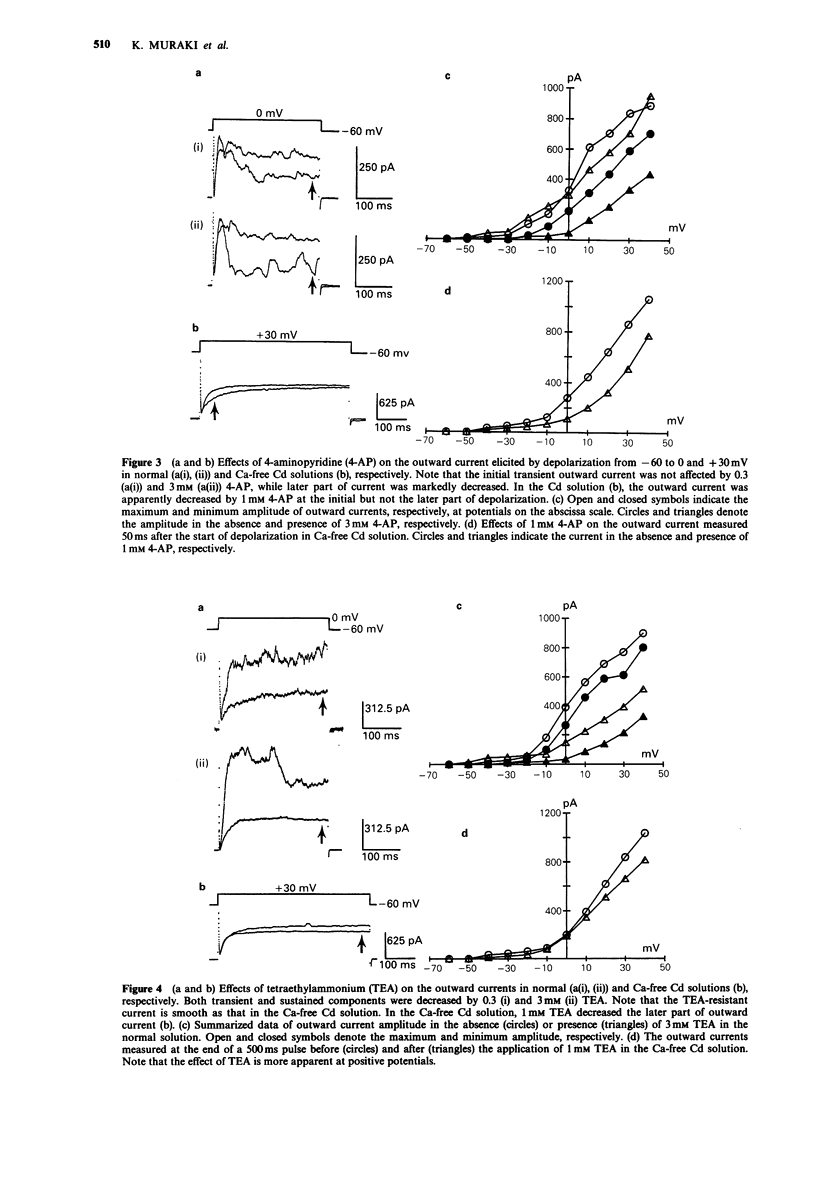
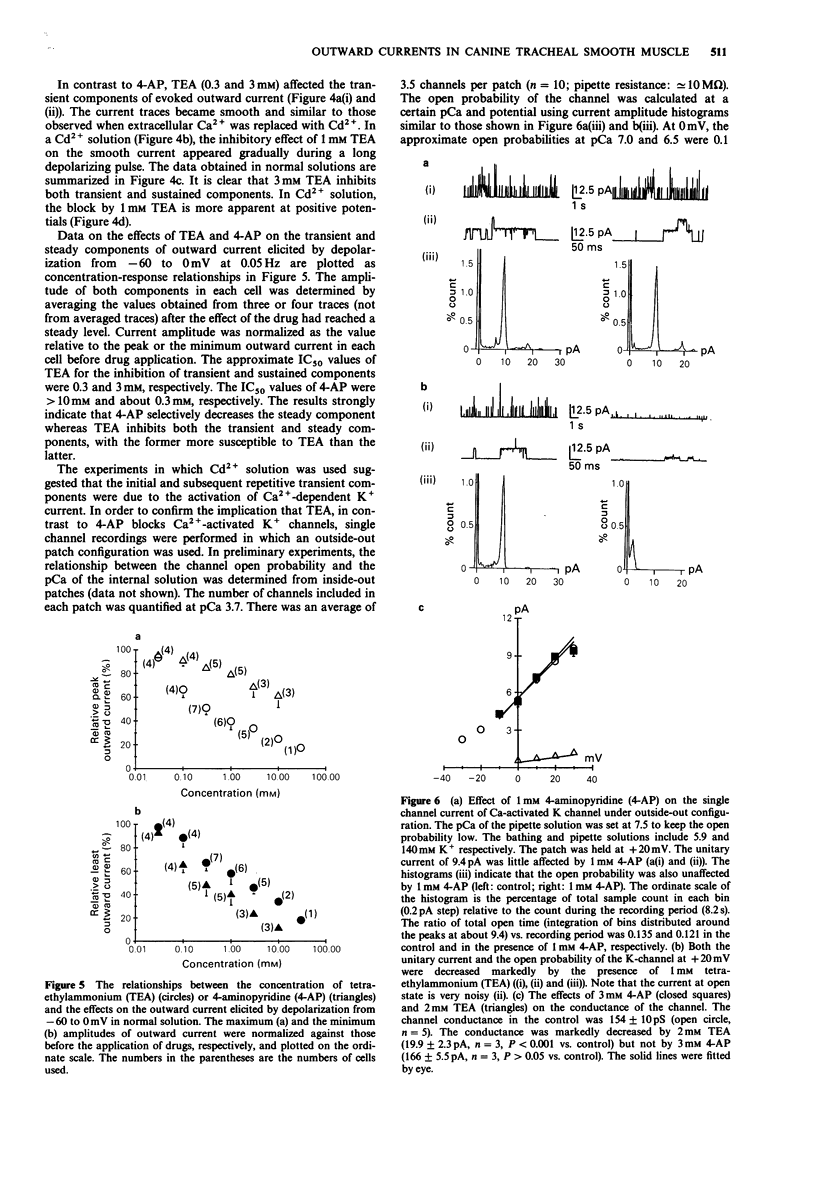
1. The effects of tetraethylammonium (TEA) and 4-aminopyridine (4-AP) on membrane currents and on single channel K currents in smooth muscle cells isolated from canine trachea were examined by use of tight seal whole cell- and patch-clamp techniques. 2. Depolarizing current applied through a recording pipette did not elicit an action potential under current clamp. A strong outward rectification was observed. 3. In most cells under voltage-clamp, only an outward current was observed upon depolarization from -60 mV when a pipette solution contained mainly KCl. The outward current consisted of three components; a large initial transient, a following sustained component and an additional component of irregular small transients on the sustained one. The two transient components were almost abolished when extracellular and pipette solutions contained 2.2 mM Cd2+ (0 mM Ca2+) and 10 mM EGTA, respectively. The sustained component was well maintained under these conditions. 4. TEA at low concentrations (less than 1 mM) effectively decreased the transient components and made the outward current smooth; it also suppressed the sustained component at higher concentrations. In outside-out patches, external 1 mM TEA reduced the single channel conductance of Ca-activated K channels by about 87% whereas 3 mM 4-AP did not. 4-AP at low concentrations (less than 3 mM) selectively reduced the sustained component of the outward current. 5. A Ca current recorded after the suppression of outward current by internal Cs+ had a peak of approximately 200 pA at +10 mV (holding potential: -60 mV). The half inactivation voltage in the steady-state was approximately -30 mV.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. The effects of tetraethylammonium ions, 4-aminopyridine or quinidine on K+-currents in single smooth muscle cells of the rabbit portal vein. Biomed Biochim Acta. 1987;46(8-9):S673–S676. [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Bregestovski P. D., Printseva OYu, Serebryakov V., Stinnakre J., Turmin A., Zamoyski V. Comparison of Ca2+-dependent K+ channels in the membrane of smooth muscle cells isolated from adult and foetal human aorta. Pflugers Arch. 1988 Nov;413(1):8–13. doi: 10.1007/BF00581222. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Yamaguchi T. Membrane potential-dependent and-independent tension in the canine tracheal muscle. J Pharmacol Exp Ther. 1977 May;201(2):276–284. [PubMed] [Google Scholar]
- Farley J. M., Miles P. R. Role of depolarization in acetylcholine-induced contractions of dog trachealis muscle. J Pharmacol Exp Ther. 1977 Apr;201(1):199–205. [PubMed] [Google Scholar]
- Fink R., Wettwer E. Modified K-channel gating by exhaustion and the block by internally applied TEA+ and 4-aminopyridine in muscle. Pflugers Arch. 1978 May 31;374(3):289–292. doi: 10.1007/BF00585607. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Potential-dependent calcium inward current in a single isolated smooth muscle cell of the guinea-pig taenia caeci. J Physiol. 1986 Nov;380:1–16. doi: 10.1113/jphysiol.1986.sp016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G., Kuhn H., Thorens S. The effect of tetraethylammonium chloride on calcium fluxes in smooth muscle from rabbit main pulmonary artery. J Physiol. 1980 Jun;303:225–241. doi: 10.1113/jphysiol.1980.sp013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kitamura K., Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980 Jan;68(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Takeda M., Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Physiol. 1989 Apr;256(4 Pt 1):C880–C885. doi: 10.1152/ajpcell.1989.256.4.C880. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Ionic currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1989 Apr;411:131–159. doi: 10.1113/jphysiol.1989.sp017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. Effect of 4-aminopyridine on potassium permeability of canine tracheal smooth muscle cell membrane. Jpn J Pharmacol. 1983 Feb;33(1):201–208. doi: 10.1254/jjp.33.201. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. Effect of procaine on potassium permeability of canine tracheal smooth muscle. Pflugers Arch. 1982 Aug;394(2):144–149. doi: 10.1007/BF00582916. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol. 1981 Jul;316:33–46. doi: 10.1113/jphysiol.1981.sp013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Inoue R., Okabe K., Kitamura K., Kuriyama H. A newly identified Ca2+ dependent K+ channel in the smooth muscle membrane of single cells dispersed from the rabbit portal vein. Pflugers Arch. 1986 Feb;406(2):138–143. doi: 10.1007/BF00586674. [DOI] [PubMed] [Google Scholar]
- Kannan M. S., Jager L. P., Daniel E. E., Garfield R. E. Effects of 4-aminopyridine and tetraethylammonium chloride on the electrical activity and cable properties of canine tracheal smooth muscle. J Pharmacol Exp Ther. 1983 Dec;227(3):706–715. [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Kotlikoff M. I. Calcium currents in isolated canine airway smooth muscle cells. Am J Physiol. 1988 Jun;254(6 Pt 1):C793–C801. doi: 10.1152/ajpcell.1988.254.6.C793. [DOI] [PubMed] [Google Scholar]
- Kroeger E. A., Stephens N. L. Effect of tetraethylammonium on tonic airway smooth muscle: initiation of phasic electrical activity. Am J Physiol. 1975 Feb;228(2):633–636. doi: 10.1152/ajplegacy.1975.228.2.633. [DOI] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Mironneau C., Mironneau J., Pacaud P. Two types of calcium currents in single smooth muscle cells from rat portal vein. J Physiol. 1989 May;412:333–349. doi: 10.1113/jphysiol.1989.sp017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N., Shigenobu K., Kasuya Y. Contractile response and electrophysiological properties in enzymatically dispersed smooth muscle cells of rat vas deferens. Pflugers Arch. 1987 Feb;408(2):112–119. doi: 10.1007/BF00581338. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Quandt F. N. Three kinetically distinct potassium channels in mouse neuroblastoma cells. J Physiol. 1988 Jan;395:401–418. doi: 10.1113/jphysiol.1988.sp016926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Small R. C. Electrical slow waves and tone of guinea-pig isolated trachealis muscle: effects of drugs and temperature changes. Br J Pharmacol. 1982 Sep;77(1):45–54. doi: 10.1111/j.1476-5381.1982.tb09267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E. A., Kromer U. Induction of a myogenic response in tonic airway smooth muscle by tetraethylammonium. Am J Physiol. 1975 Feb;228(2):628–632. doi: 10.1152/ajplegacy.1975.228.2.628. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E. Effect of hypoxia on airway smooth muscle mechanics and electrophysiology. J Appl Physiol. 1970 May;28(5):630–635. doi: 10.1152/jappl.1970.28.5.630. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L., Stefani E. Ca2+ and K+ current in cultured vascular smooth muscle cells from rat aorta. Pflugers Arch. 1987 Apr;408(4):417–419. doi: 10.1007/BF00581139. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Identification and characterization of major ionic currents in isolated smooth muscle cells using the voltage-clamp technique. Pflugers Arch. 1987 Feb;408(2):83–97. doi: 10.1007/BF00581336. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Outward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):551–564. doi: 10.1085/jgp.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M., Someya T., Nishio A., Yabu H. Whole-cell and unitary Ca channel currents in mammalian intestinal smooth muscle cells: evidence for the existence of two types of Ca channels. Pflugers Arch. 1988 Feb;411(2):229–231. doi: 10.1007/BF00582322. [DOI] [PubMed] [Google Scholar]


