Abstract
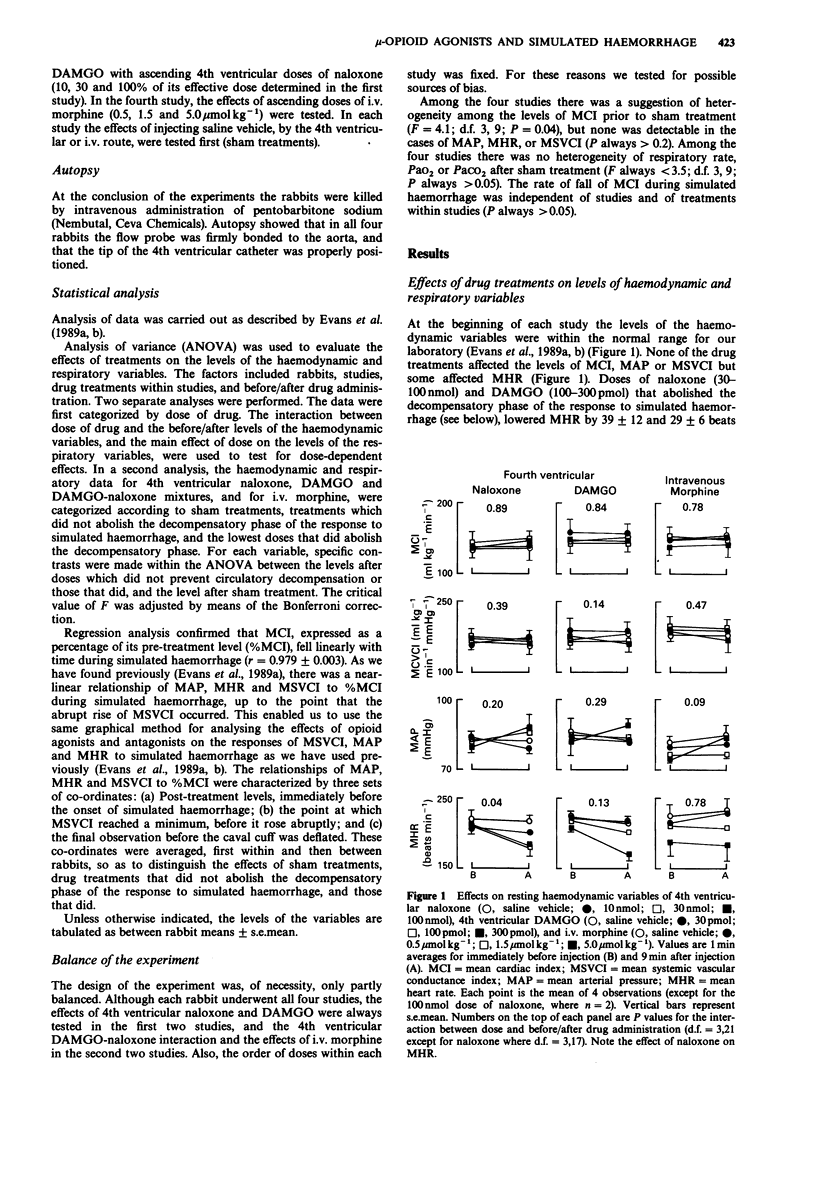
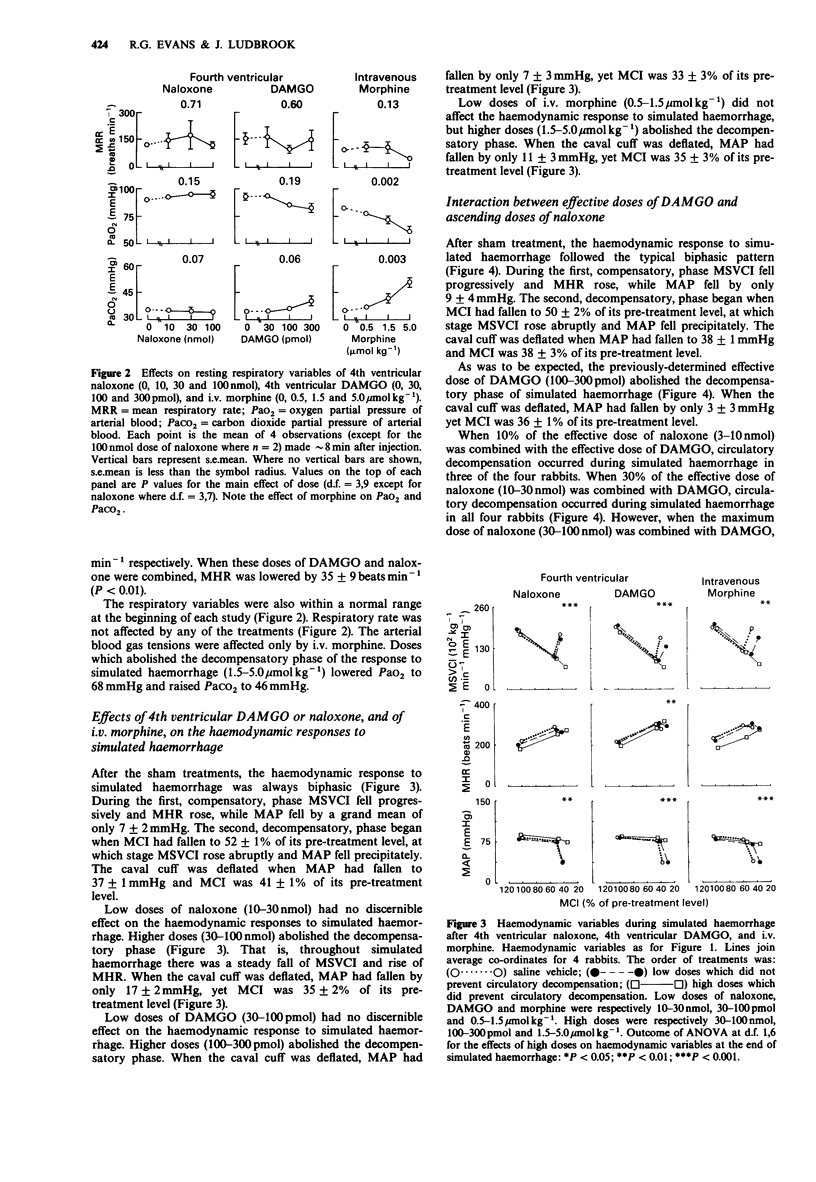
1. Cardiac output, arterial pressure, heart rate, systemic vascular conductance, respiratory rate and arterial blood PO2 and PCO2 were measured in unanaesthetized rabbits. Haemorrhage was simulated by inflating a cuff placed around the inferior vena cava so that cardiac output fell at a constant rate of about 8% of its resting value per min. 2. The effects of drug treatments on resting haemodynamic and respiratory variables, and on the haemodynamic response to simulated haemorrhage, were tested. The treatments were; 4th ventricular (-)-naloxone HCl (10-100 nmol), 4th ventricular H-Tyr-D-Ala-Gly-MePhe-NH(CH2)2OH (DAMGO; 30-300 pmol), and i.v. morphine sulphate (0.5-5.0 mumol kg-1). The interactions of graded 4th ventricular doses of naloxone (3-100 nmol) with the actions of DAMGO (100-300 pmol) on these responses were also assessed. 3. After sham treatments, the circulatory response to simulated haemorrhage had two phases. During the first compensatory phase, systemic vascular conductance fell, heart rate rose, and mean arterial pressure fell by only about 7 mmHg. A second decompensatory phase supervened when cardiac output had fallen by about 50%. At this point systemic vascular conductance rose abruptly and arterial pressure fell to less than or equal to 40 mmHg. 4. Low 4th ventricular doses of naloxone (10-30 nmol) and DAMGO (30-100 pmol) had no discernible effect on the circulatory response to simulated haemorrhage. Higher doses of naloxone (30-100 nmol) and DAMGO (100-300 pmol) prevented the decompensatory phase. These high doses of naloxone and DAMGO lowered resting heart rate without affecting the other haemodynamic or respiratory variables.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke S. L., Dorward P. K. Influence of endogenous opiates and cardiac afferents on renal nerve activity during haemorrhage in conscious rabbits. J Physiol. 1988 Aug;402:9–27. doi: 10.1113/jphysiol.1988.sp017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato R., Holaday J. W. Multiple opioid receptors in endotoxic shock: evidence for delta involvement and mu-delta interactions in vivo. Proc Natl Acad Sci U S A. 1984 May;81(9):2898–2901. doi: 10.1073/pnas.81.9.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood M. R., Muddle J. R., Spyer K. M. Opiate receptor subtypes in the nucleus tractus solitarii of the cat: the effect of vagal section. Eur J Pharmacol. 1988 Oct 11;155(1-2):85–92. doi: 10.1016/0014-2999(88)90405-0. [DOI] [PubMed] [Google Scholar]
- Evans R. G., Ludbrook J., Van Leeuwen A. F. Role of central opiate receptor subtypes in the circulatory responses of awake rabbits to graded caval occlusions. J Physiol. 1989 Dec;419:15–31. doi: 10.1113/jphysiol.1989.sp017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris I. B., Iannos J., Jamieson G. G., Ludbrook J. The circulatory effects of acute hypervolemia and hemodilution in conscious rabbits. Circ Res. 1981 Jun;48(6 Pt 1):825–834. doi: 10.1161/01.res.48.6.825. [DOI] [PubMed] [Google Scholar]
- Flórez J., McCarthy L. E., Borison H. L. A comparative study in the cat of the respiratory effects of morphine injected intravenously and into the cerebrospinal fluid. J Pharmacol Exp Ther. 1968 Oct;163(2):448–455. [PubMed] [Google Scholar]
- Handa B. K., Land A. C., Lord J. A., Morgan B. A., Rance M. J., Smith C. F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981 Apr 9;70(4):531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Holaday J. W., Ruvio B. A., Robles L. E., Johnson C. E., D'Amato R. J. M 154,129, a putative delta antagonist, reverses endotoxic shock without altering morphine analgesia. Life Sci. 1982 Nov 15;31(20-21):2209–2212. doi: 10.1016/0024-3205(82)90120-5. [DOI] [PubMed] [Google Scholar]
- Jordan D., Spyer K. M. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Korner P. I. The role of the arterial chemoreceptors and baroreceptors in the circulatory response to hypoxia of the rabbit. J Physiol. 1965 Sep;180(2):279–303. doi: 10.1113/jphysiol.1965.sp007703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook J., Graham W. F. The role of cardiac receptor and arterial baroreceptor reflexes in control of the circulation during acute change of blood volume in the conscious rabbit. Circ Res. 1984 Apr;54(4):424–435. doi: 10.1161/01.res.54.4.424. [DOI] [PubMed] [Google Scholar]
- Ludbrook J., Potocnik S. J., Woods R. L. Simulation of acute haemorrhage in unanaesthetized rabbits. Clin Exp Pharmacol Physiol. 1988 Aug;15(8):575–584. doi: 10.1111/j.1440-1681.1988.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Ludbrook J., Rutter P. C. Effect of naloxone on haemodynamic responses to acute blood loss in unanaesthetized rabbits. J Physiol. 1988 Jun;400:1–14. doi: 10.1113/jphysiol.1988.sp017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan J., Paterson S. J., Tavani A., Kosterlitz H. W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988 Jul;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- May C. N., Dashwood M. R., Whitehead C. J., Mathias C. J. Differential cardiovascular and respiratory responses to central administration of selective opioid agonists in conscious rabbits: correlation with receptor distribution. Br J Pharmacol. 1989 Nov;98(3):903–913. doi: 10.1111/j.1476-5381.1989.tb14620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. N., Whitehead C. J., Heslop K. E., Mathias C. J. Evidence that intravenous morphine stimulates central opiate receptors to increase sympatho-adrenal outflow and cause hypertension in conscious rabbits. Clin Sci (Lond) 1989 Apr;76(4):431–437. doi: 10.1042/cs0760431. [DOI] [PubMed] [Google Scholar]
- Miller L., Shaw J. S., Whiting E. M. The contribution of intrinsic activity to the action of opioids in vitro. Br J Pharmacol. 1986 Mar;87(3):595–601. doi: 10.1111/j.1476-5381.1986.tb10202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales N., Riche D., Roques B. P., Denavit-Saubie M. Localization of mu- and delta-opioid receptors in cat respiratory areas: an autoradiographic study. Brain Res. 1985 Oct 7;344(2):382–386. doi: 10.1016/0006-8993(85)90820-0. [DOI] [PubMed] [Google Scholar]
- Schadt J. C., McKown M. D., McKown D. P., Franklin D. Hemodynamic effects of hemorrhage and subsequent naloxone treatment in conscious rabbits. Am J Physiol. 1984 Sep;247(3 Pt 2):R497–R505. doi: 10.1152/ajpregu.1984.247.3.R497. [DOI] [PubMed] [Google Scholar]
- Sheehan M. J., Hayes A. G., Tyers M. B. Pharmacology of delta-opioid receptors in the hamster vas deferens. Eur J Pharmacol. 1986 Oct 14;130(1-2):57–64. doi: 10.1016/0014-2999(86)90183-4. [DOI] [PubMed] [Google Scholar]
- Smith C. F. 16-Me cyprenorphine (RX 8008M): a potent opioid antagonist with some delta selectivity. Life Sci. 1987 Jan 19;40(3):267–274. doi: 10.1016/0024-3205(87)90342-0. [DOI] [PubMed] [Google Scholar]


