Abstract
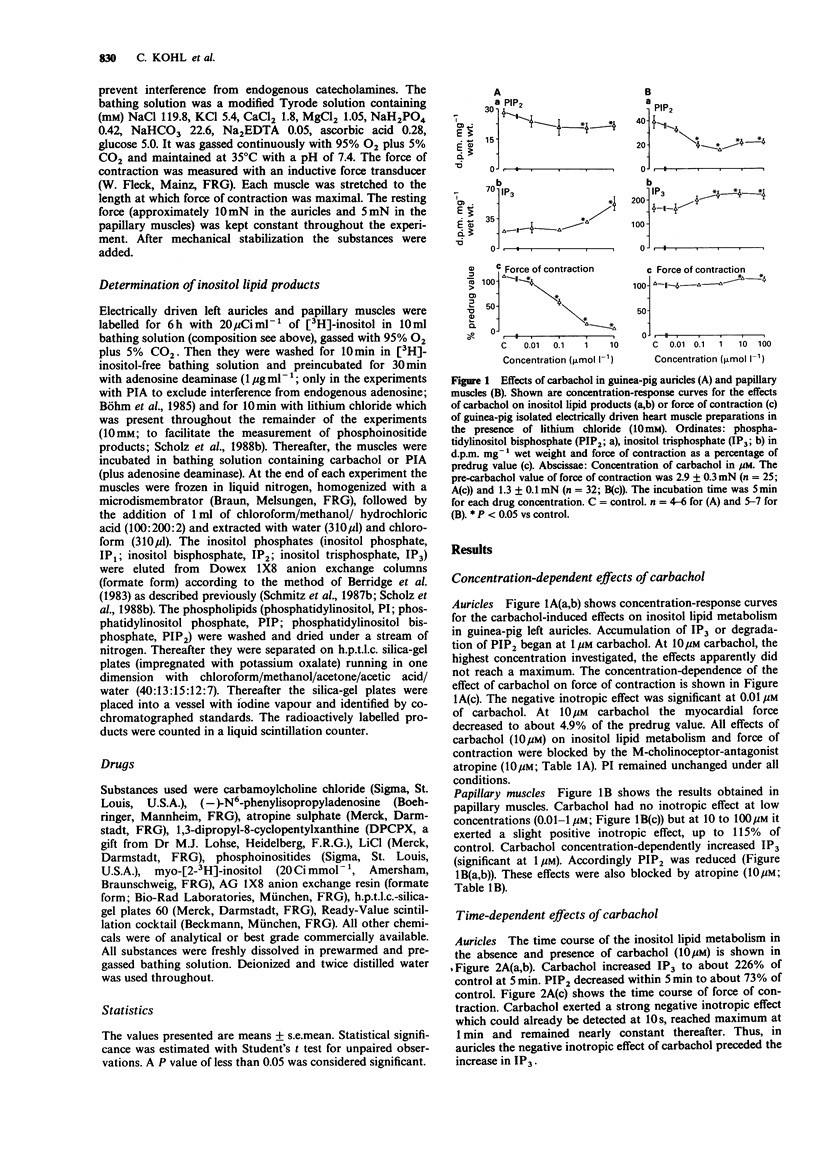
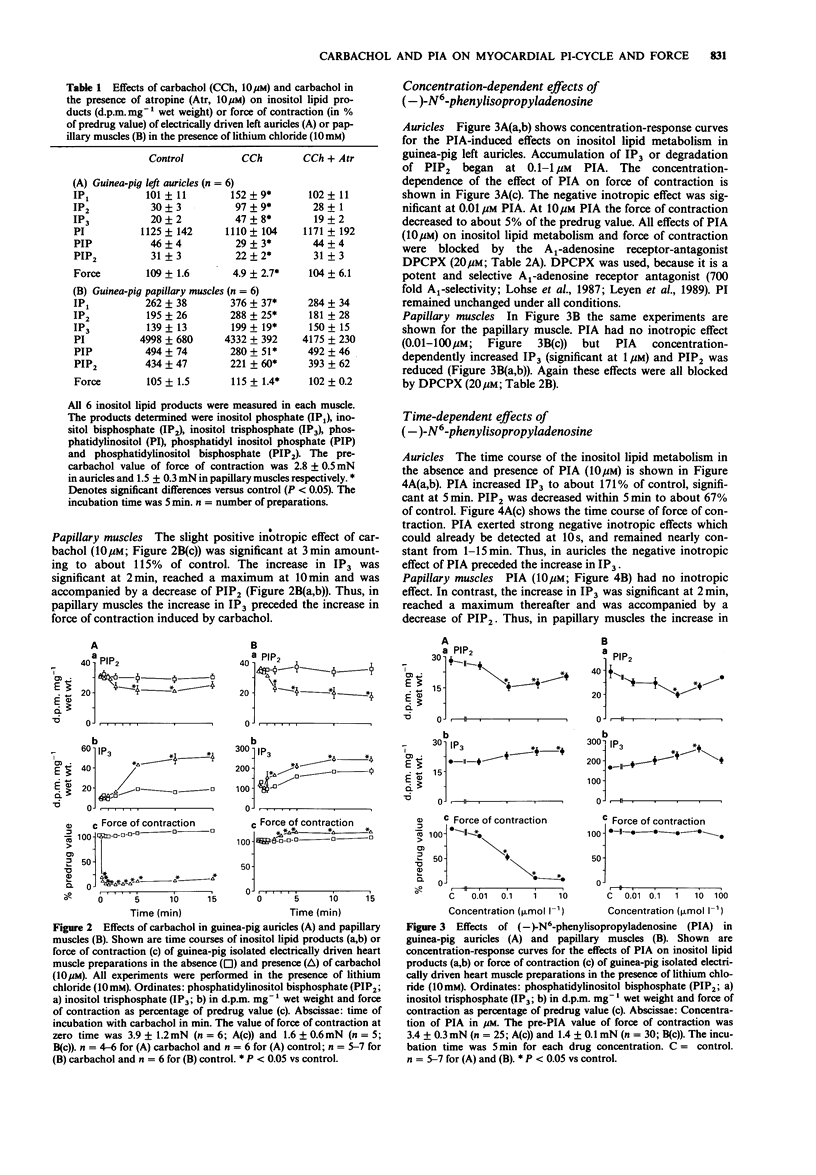
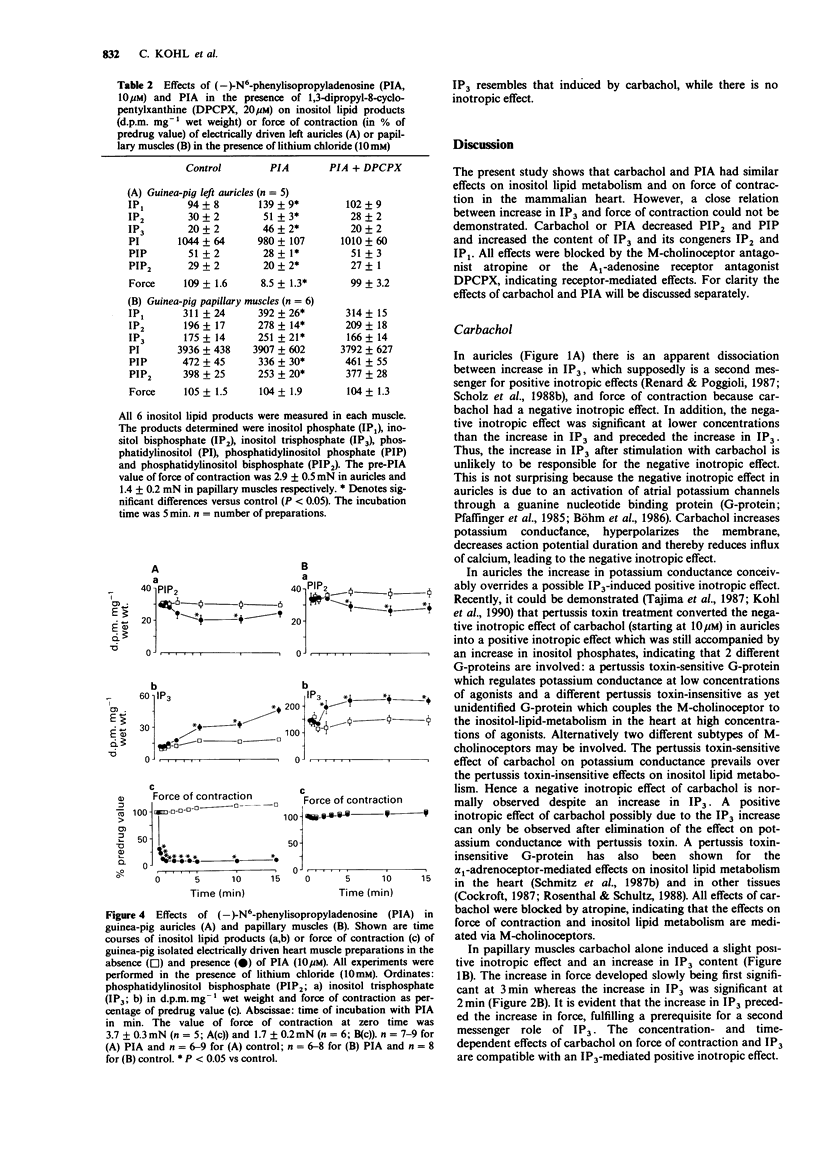
1. The effects of carbachol and the A1-adenosine receptor agonist (-)-N6-phenylisopropyladenosine (PIA) on force of contraction and inositol lipid metabolism were studied in electrically driven left auricles and papillary muscles isolated from guinea-pig hearts. Both carbachol and PIA (0.01-10 microM) had concentration-dependent negative inotropic effects in auricles. In papillary muscles PIA had no inotropic effect. Carbachol also had no inotropic effect at low concentrations (0.01-1 microM) but at 10-100 microM it exerted a slight positive inotropic effect. 2. In auricles and papillary muscles both carbachol and PIA concentration-dependently increased inositol trisphosphate (IP3; significant at 1 microM). Accordingly phosphatidylinositol bisphosphate (PIP2), the precursor of IP3, was reduced. All effects of carbachol and PIA were antagonized by atropine (10 microM) and 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 20 microM) respectively, indicating receptor-mediated effects. 3. In auricles the negative inotropic effects of carbachol and PIA preceded the increase in IP3. 4. In papillary muscles the increase in IP3 preceded the slight positive inotropic effect of carbachol, indicating that the M-cholinoceptor-mediated increase in IP3 and force of contraction may be related. However, PIA showed a comparable increase in IP3 but no inotropic effect, indicating a dissociation between those parameters. 5. In conclusion, in previous studies a close relation between increases in IP3 and force of contraction has been shown after alpha 1-adrenoceptor stimulation. The present study with carbachol supports this view. However, the present data for PIA could not show such a close relationship, questioning the role of IP3 as an endogenous regulator of force of contraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- Brown S. L., Brown J. H. Muscarinic stimulation of phosphatidylinositol metabolism in atria. Mol Pharmacol. 1983 Nov;24(3):351–356. [PubMed] [Google Scholar]
- Brückner R., Fenner A., Meyer W., Nobis T. M., Schmitz W., Scholz H. Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J Pharmacol Exp Ther. 1985 Sep;234(3):766–774. [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983 Sep 10;258(17):10233–10239. [PubMed] [Google Scholar]
- Böhm M., Brückner R., Hackbarth I., Haubitz B., Linhart R., Meyer W., Schmidt B., Schmitz W., Scholz H. Adenosine inhibition of catecholamine-induced increase in force of contraction in guinea-pig atrial and ventricular heart preparations. Evidence against a cyclic AMP- and cyclic GMP-dependent effect. J Pharmacol Exp Ther. 1984 Aug;230(2):483–492. [PubMed] [Google Scholar]
- Böhm M., Brückner R., Meyer W., Nose M., Schmitz W., Scholz H., Starbatty J. Evidence for adenosine receptor-mediated isoprenaline-antagonistic effects of the adenosine analogs PIA and NECA on force of contraction in guinea-pig atrial and ventricular cardiac preparations. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):131–139. doi: 10.1007/BF00634229. [DOI] [PubMed] [Google Scholar]
- Böhm M., Brückner R., Neumann J., Schmitz W., Scholz H., Starbatty J. Role of guanine nucleotide-binding protein in the regulation by adenosine of cardiac potassium conductance and force of contraction. Evaluation with pertussis toxin. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):403–405. doi: 10.1007/BF00500095. [DOI] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., Barsotti R. J., Lea T. J., Mulligan I. P., Patel J. R., Ferenczi M. A. Calcium release from cardiac sarcoplasmic reticulum induced by photorelease of calcium or Ins(1,4,5)P3. Am J Physiol. 1990 Feb;258(2 Pt 2):H610–H615. doi: 10.1152/ajpheart.1990.258.2.H610. [DOI] [PubMed] [Google Scholar]
- Kohl C., Schmitz W., Scholz H., Scholz J., Tóth M., Döring V., Kalmár P. Evidence for alpha 1-adrenoceptor-mediated increase of inositol trisphosphate in the human heart. J Cardiovasc Pharmacol. 1989 Feb;13(2):324–327. doi: 10.1097/00005344-198902000-00023. [DOI] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E., Johnston C. I., Woodcock E. A. Stimulation of phosphatidylinositol metabolism in atrial and ventricular myocytes. Life Sci. 1986 Dec 8;39(23):2215–2220. doi: 10.1016/0024-3205(86)90399-1. [DOI] [PubMed] [Google Scholar]
- Linden J., Hollen C. E., Patel A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ Res. 1985 May;56(5):728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- Movsesian M. A., Thomas A. P., Selak M., Williamson J. R. Inositol trisphosphate does not release Ca2+ from permeabilized cardiac myocytes and sarcoplasmic reticulum. FEBS Lett. 1985 Jun 17;185(2):328–332. doi: 10.1016/0014-5793(85)80932-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nosek T. M., Williams M. F., Zeigler S. T., Godt R. E. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Quist E. E. Evidence for a carbachol stimulated phosphatidylinositol effect in heart. Biochem Pharmacol. 1982 Oct 1;31(19):3130–3133. doi: 10.1016/0006-2952(82)90095-8. [DOI] [PubMed] [Google Scholar]
- Renard D., Poggioli J. Does the inositol tris/tetrakisphosphate pathway exist in rat heart? FEBS Lett. 1987 Jun 8;217(1):117–123. doi: 10.1016/0014-5793(87)81254-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Schultz G. Guaninnucleotid-bindende Proteine als membranäre Signaltransduktionskomponenten und Regulatoren enzymatischer Effektoren. Klin Wochenschr. 1988 Jun 15;66(12):511–523. doi: 10.1007/BF01736519. [DOI] [PubMed] [Google Scholar]
- Schmitz W., Scholz H., Scholz J., Steinfath M. Increase in IP3 precedes alpha-adrenoceptor-induced increase in force of contraction in cardiac muscle. Eur J Pharmacol. 1987 Aug 4;140(1):109–111. doi: 10.1016/0014-2999(87)90641-8. [DOI] [PubMed] [Google Scholar]
- Schmitz W., Scholz H., Scholz J., Steinfath M., Lohse M., Puurunen J., Schwabe U. Pertussis toxin does not inhibit the alpha 1-adrenoceptor-mediated effect on inositol phosphate production in the heart. Eur J Pharmacol. 1987 Feb 24;134(3):377–378. doi: 10.1016/0014-2999(87)90374-8. [DOI] [PubMed] [Google Scholar]
- Scholz J. Inositoltrisphosphat, ein neuer "Second Messenger" für positiv inotrope Wirkungen am Herzen? Klin Wochenschr. 1989 Mar 1;67(5):271–279. doi: 10.1007/BF01892894. [DOI] [PubMed] [Google Scholar]
- Scholz J., Schaefer B., Schmitz W., Scholz H., Steinfath M., Lohse M., Schwabe U., Puurunen J. Alpha-1 adrenoceptor-mediated positive inotropic effect and inositol trisphosphate increase in mammalian heart. J Pharmacol Exp Ther. 1988 Apr;245(1):327–335. [PubMed] [Google Scholar]
- Tajima T., Tsuji Y., Brown J. H., Pappano A. J. Pertussis toxin-insensitive phosphoinositide hydrolysis, membrane depolarization, and positive inotropic effect of carbachol in chick atria. Circ Res. 1987 Sep;61(3):436–445. doi: 10.1161/01.res.61.3.436. [DOI] [PubMed] [Google Scholar]
- von der Leyen H., Schmitz W., Scholz H., Scholz J., Lohse M. J., Schwabe U. Effects of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), a highly selective adenosine receptor antagonist, on force of contraction in guinea-pig atrial and ventricular cardiac preparations. Naunyn Schmiedebergs Arch Pharmacol. 1989 Aug;340(2):204–209. doi: 10.1007/BF00168970. [DOI] [PubMed] [Google Scholar]


