Abstract
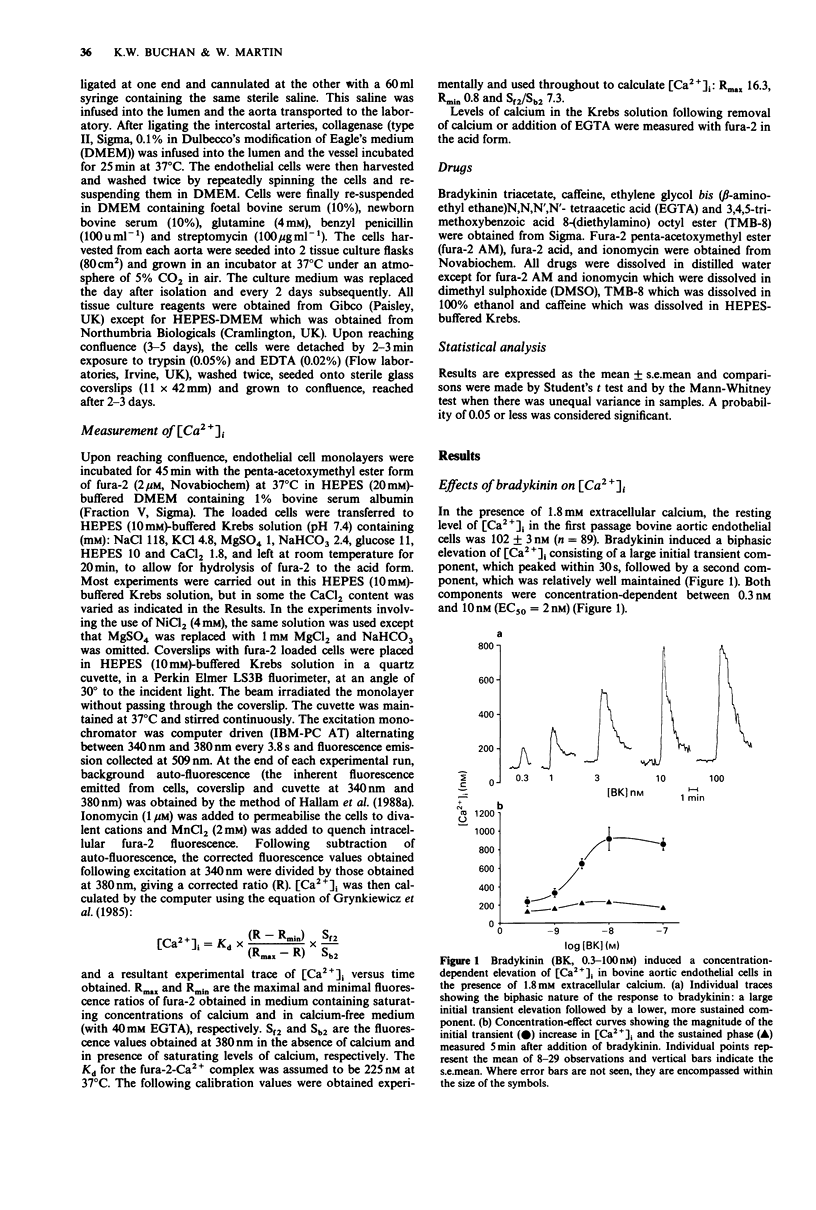
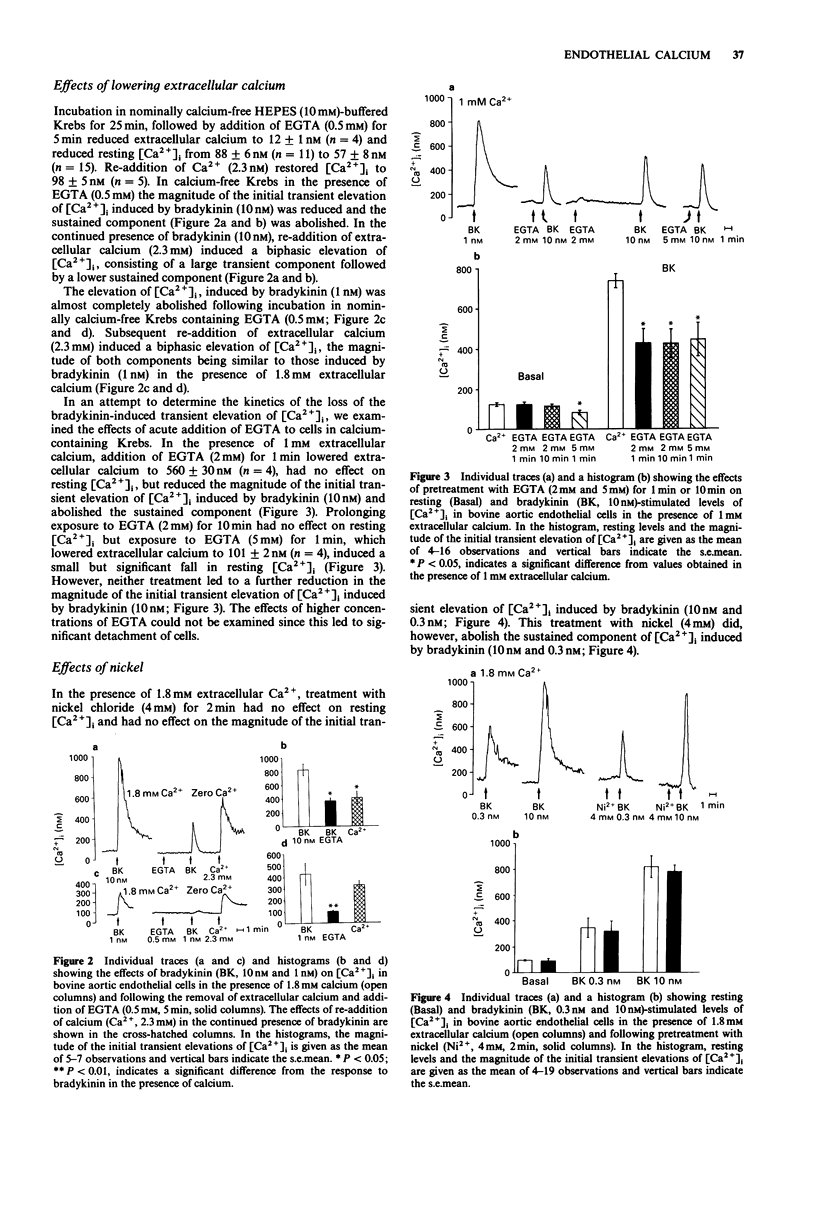
1. In the presence of 1.8 mM extracellular calcium, bradykinin (0.3 nM-100 nM) induced a biphasic elevation of intracellular calcium ([Ca2+]i) in bovine aortic endothelial cells, consisting of an initial, large transient component followed by a lower sustained component. 2. When endothelial cells were bathed in nominally calcium-free solution containing 0.5 mM EGTA, bradykinin induced only a transient elevation of [Ca2+]i: the magnitude of this was significantly smaller than that obtained in the presence of extracellular calcium and the sustained phase was abolished. In the continued presence of bradykinin, re-addition of extracellular calcium to achieve a level of around 1.8 mM resulted in the induction of a biphasic elevation of [Ca2+]i consisting of a large initial component followed by a lower sustained component. 3. In the presence of 1.8 mM extracellular calcium, caffeine (5 mM) induced a small elevation of [Ca2+]i. When endothelial cells were bathed in nominally calcium-free solution containing 0.5 mM EGTA, the caffeine-induced elevation of [Ca2+]i was almost completely abolished. 4. In the presence of 1.8 mM extracellular calcium, treatment of endothelial cells with the calcium influx blocker, nickel chloride (4 mM), had no effect on resting [Ca2+]i or on the magnitude of the bradykinin-induced initial transient elevation of [Ca2+]i but abolished the sustained component. 5. In the presence of 1 mM extracellular calcium, treatment with the calcium chelator EGTA (2 mM; 1 min) had no effect on resting [Ca2+]i but the magnitude of the bradykinin-induced initial transient elevation of [Ca2+]i was significantly reduced.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. A., Capasso E. A. Thrombin and histamine activate phospholipase C in human endothelial cells via a phorbol ester-sensitive pathway. J Cell Physiol. 1988 Jul;136(1):54–62. doi: 10.1002/jcp.1041360107. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Aglietta M., Sanavio F., Stacchini A., Lauri D., Camussi G. Alkyl-ether phosphoglycerides influence calcium fluxes into human endothelial cells. J Immunol. 1985 Oct;135(4):2748–2753. [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Zeh R., Busse R. Desensitization of the bradykinin-induced rise in intracellular free calcium in cultured endothelial cells. Pflugers Arch. 1988 Oct;412(6):654–658. doi: 10.1007/BF00583768. [DOI] [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscles. Eur J Pharmacol. 1974 Jun;27(1):25–33. doi: 10.1016/0014-2999(74)90198-8. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Avdonin P. V., Posin E. Y., Popov E. G., Danilov S. M., Tkachuk V. A. Influence of vasoactive agents on cytoplasmic free calcium in vascular endothelial cells. J Appl Physiol (1985) 1988 Nov;65(5):2221–2227. doi: 10.1152/jappl.1988.65.5.2221. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Ritchie A. K., Navarro L. T., Eskin S. G. Bradykinin-stimulated calcium influx in cultured bovine aortic endothelial cells. Am J Physiol. 1988 Aug;255(2 Pt 2):H219–H227. doi: 10.1152/ajpheart.1988.255.2.H219. [DOI] [PubMed] [Google Scholar]
- Seid J. M., MacNeil S., Tomlinson S. Calcium, calmodulin, and the production of prostacyclin by cultured vascular endothelial cells. Biosci Rep. 1983 Nov;3(11):1007–1015. doi: 10.1007/BF01121027. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. G., Martin W. Differential control and calcium-dependence of production of endothelium-derived relaxing factor and prostacyclin by pig aortic endothelial cells. Br J Pharmacol. 1989 Jul;97(3):683–690. doi: 10.1111/j.1476-5381.1989.tb12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


