Abstract
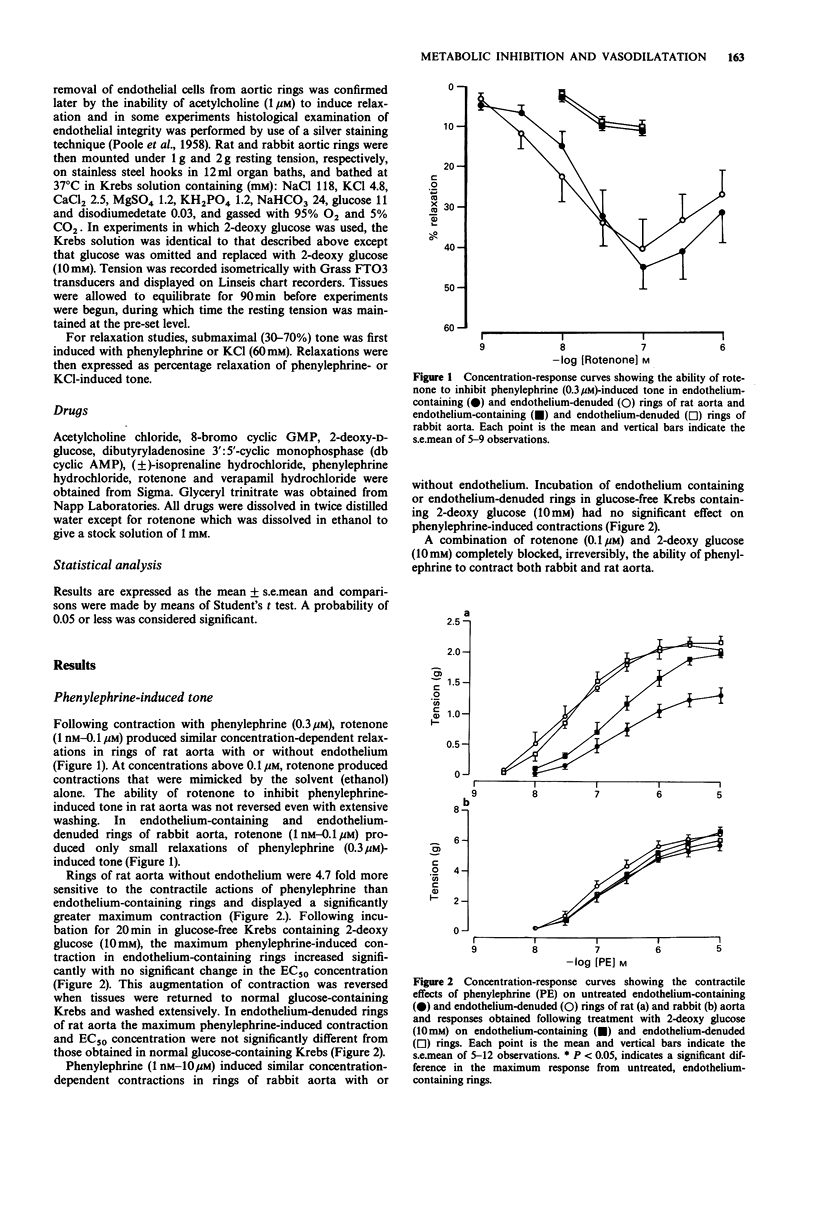
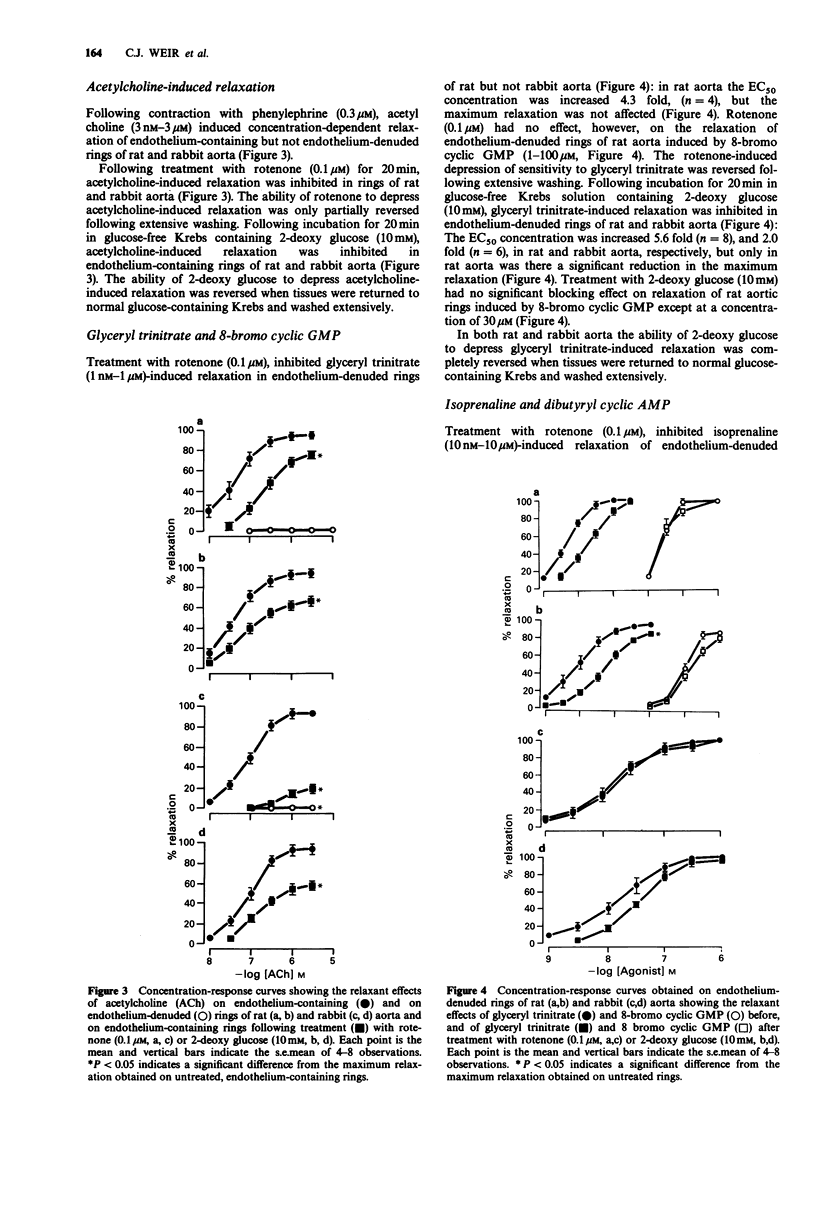
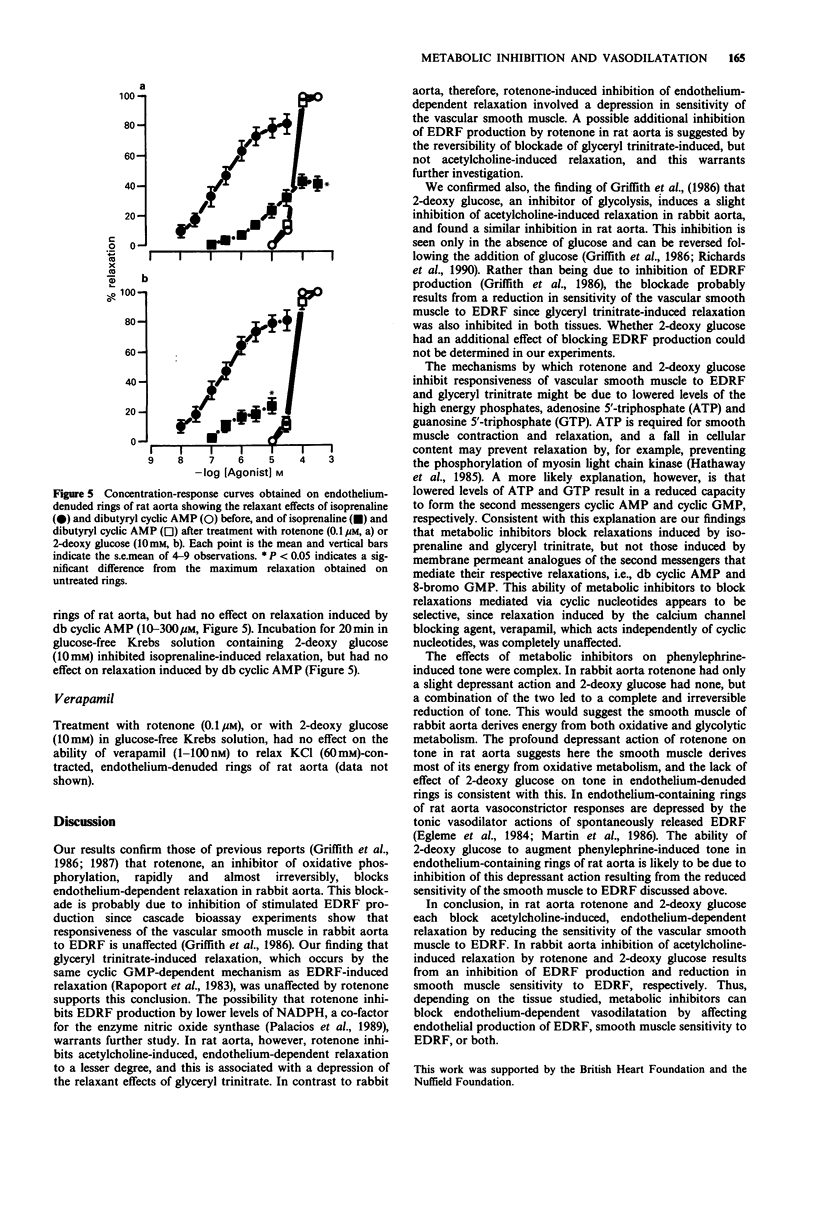
1. Basal release of endothelium-derived relaxing factor (EDRF) rendered endothelium-containing rings of rat aorta 4.7 fold less sensitive to the contractile actions of phenylephrine and depressed the maximum response when compared with endothelium-denuded rings. The responsiveness and maximum response to phenylephrine was, however, similar in rings of rabbit aorta with or without endothelium. 2. Rotenone (1 nM-0.1 microM), an inhibitor of oxidative phosphorylation, induced a profound, irreversible blockade of phenylephrine-induced tone in endothelium-containing and endothelium-denuded rings of rat aorta, but induced only slight inhibition of tone in rings of rabbit aorta. 3. 2-Deoxy glucose (10 mM), an inhibitor of glycolysis, had no effect on phenylephrine-induced contraction in endothelium-denuded rings of rat aorta, but inhibited reversibly the endothelium-dependent depression of contraction in endothelium containing rings. 2-Deoxy glucose had no effect on phenylephrine-induced contraction in rings of rabbit aorta with or without endothelium. 4. Rotenone (0.1 microM) inhibited acetylcholine-induced, endothelium-dependent relaxation of phenylephrine-contracted rings or rat and rabbit aorta. In endothelium-denuded rings of rat aorta, relaxation induced by glyceryl trinitrate of isoprenaline was also inhibited, but relaxation induced by 8-bromo cyclic GMP or dibutyryl cyclic AMP was not. Relaxation induced by verapamil on KCl-contracted, endothelium-denuded rings of rat aorta was also unaffected. 5. 2-Deoxy glucose (10 mM) inhibited acetylcholine-induced, endothelium-dependent relaxation of phenylephrine-contracted rings of rat and rabbit aorta.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eglème C., Godfraind T., Miller R. C. Enhanced responsiveness of rat isolated aorta to clonidine after removal of the endothelial cells. Br J Pharmacol. 1984 Jan;81(1):16–18. doi: 10.1111/j.1476-5381.1984.tb10736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Henderson A. H. Unstimulated release of endothelium derived relaxing factor is independent of mitochondrial ATP generation. Cardiovasc Res. 1987 Aug;21(8):565–568. doi: 10.1093/cvr/21.8.565. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Konicki M. V., Coolican S. A. Phosphorylation of myosin light chain kinase from vascular smooth muscle by cAMP- and cGMP-dependent protein kinases. J Mol Cell Cardiol. 1985 Sep;17(9):841–850. doi: 10.1016/s0022-2828(85)80098-5. [DOI] [PubMed] [Google Scholar]
- Holtz J., Förstermann U., Pohl U., Giesler M., Bassenge E. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1161–1169. [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Richards J. M., Gibson I. F., Martin W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]


