Abstract
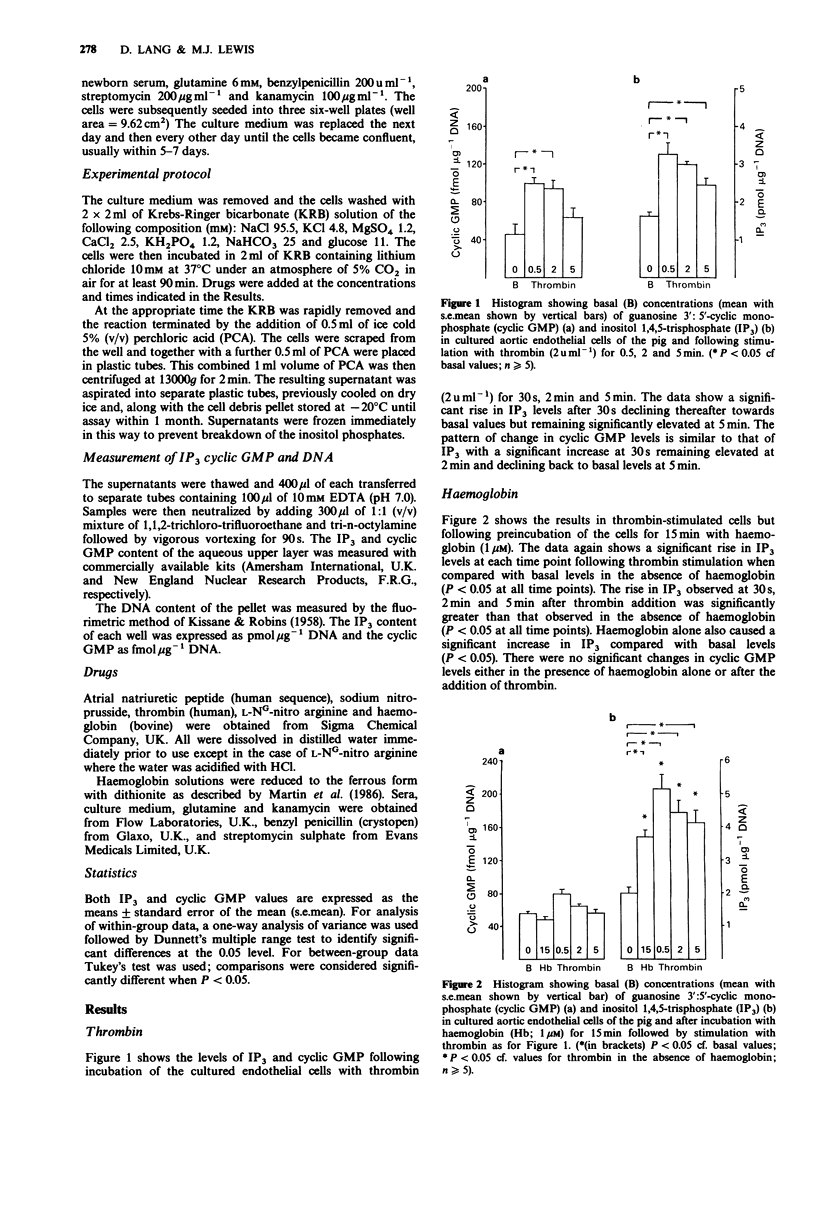
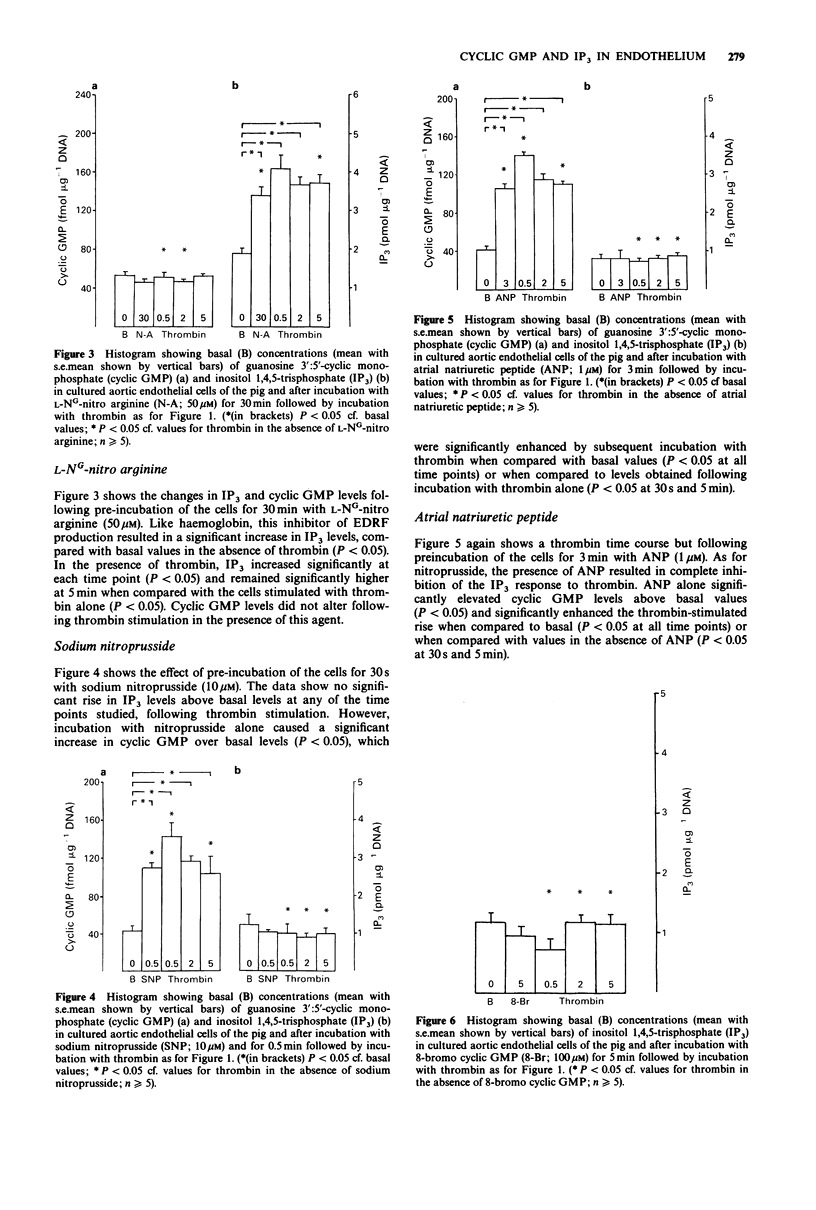
1. In cultured endothelial cells of the pig the endothelium-derived relaxing factor (EDRF) releasing agent thrombin (2 u ml-1) caused a significant increase in basal levels of both guanosine 3':5'-cyclic monophosphate (cyclic GMP) and inositol 1,4,5-trisphosphate (IP3). This increase was time dependent, with peak levels occurring at 2 min and returning towards basal values after 5 min. 2. Pretreatment of the cells with the EDRF inhibitors haemoglobin (1 microM) or L-NG-nitro arginine (50 microM) significantly reduced the cyclic GMP response to thrombin. Both agents also resulted in significant elevations in basal levels of IP3. The IP3 response to thrombin was significantly enhanced at all time points by haemoglobin and at 5 min for L-NG-nitro arginine, when compared with the response to thrombin alone. 3. Pretreatment of the cells with either sodium nitroprusside (10 microM) or atrial natriuretic peptide (1 microM) caused a significant elevation of basal cyclic GMP levels. Although subsequent exposure to thrombin caused a further increase in cyclic GMP, which together with the rise induced by the previous two agents was significantly greater than the increase caused by thrombin alone, the incremental increase induced by thrombin was markedly less in the presence of nitroprusside or atrial natriuretic peptide. Both these agents, as well as 8-bromo cyclic GMP, resulted in a significant suppression of the IP3 response to thrombin. 4. These findings show that one mechanism for the inhibitory effect of cyclic GMP on EDRF release from endothelium may be through the inhibition of IP3 formation in response to EDRF releasing agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Cocks T. M. Endothelium-derived relaxing factor. Pharmacol Ther. 1989;41(1-2):303–352. doi: 10.1016/0163-7258(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Collins P., Griffith T. M., Henderson A. H., Lewis M. J. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986 Dec;381:427–437. doi: 10.1113/jphysiol.1986.sp016336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Evans H. G., Smith J. A., Lewis M. J. Release of endothelium-derived relaxing factor is inhibited by 8-bromo-cyclic guanosine monophosphate. J Cardiovasc Pharmacol. 1988 Dec;12(6):672–677. doi: 10.1097/00005344-198812000-00008. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., Shimokawa H., Vanhoutte P. M. Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J Physiol. 1989 Jan;408:549–560. doi: 10.1113/jphysiol.1989.sp017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Hogan J. C., Smith J. A., Richards A. C., Lewis M. J. Atrial natriuretic peptide inhibits the release of endothelium-derived relaxing factor from blood vessels of the rabbit. Eur J Pharmacol. 1989 Jun 8;165(1):129–134. doi: 10.1016/0014-2999(89)90778-4. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea-pig taenia coli. Br J Pharmacol. 1984 Feb;81(2):393–400. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lang D., Lewis M. J. Endothelium-derived relaxing factor inhibits the formation of inositol trisphosphate by rabbit aorta. J Physiol. 1989 Apr;411:45–52. doi: 10.1113/jphysiol.1989.sp017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb A. L., Izzo N. J., Jr, Johnson R. M., Garrison J. C., Peach M. J. Endothelium-derived relaxing factor release associated with increased endothelial cell inositol trisphosphate and intracellular calcium. Am J Cardiol. 1988 Oct 5;62(11):36G–40G. doi: 10.1016/0002-9149(88)90030-6. [DOI] [PubMed] [Google Scholar]
- Martin W., Smith J. A., White D. G. The mechanisms by which haemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitric oxide, or bovine retractor penis inhibitory factor. Br J Pharmacol. 1986 Nov;89(3):563–571. doi: 10.1111/j.1476-5381.1986.tb11157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985 Jun;233(3):679–685. [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Moreno F., García-Barreno P. Mitogenic activity and inositide metabolism in thrombin-stimulated pig aorta endothelial cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1302–1309. doi: 10.1016/0006-291x(87)91579-8. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Henderson A. H. Stimulus-secretion coupling in vascular endothelial cells. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Pollock W. K., Wreggett K. A., Irvine R. F. Inositol phosphate production and Ca2+ mobilization in human umbilical-vein endothelial cells stimulated by thrombin and histamine. Biochem J. 1988 Dec 1;256(2):371–376. doi: 10.1042/bj2560371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [PubMed] [Google Scholar]
- Ryan U. S., Avdonin P. V., Posin E. Y., Popov E. G., Danilov S. M., Tkachuk V. A. Influence of vasoactive agents on cytoplasmic free calcium in vascular endothelial cells. J Appl Physiol (1985) 1988 Nov;65(5):2221–2227. doi: 10.1152/jappl.1988.65.5.2221. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Lang D. Release of endothelium-derived relaxing factor from pig cultured aortic endothelial cells, as assessed by changes in endothelial cell cyclic GMP content, is inhibited by a phorbol ester. Br J Pharmacol. 1990 Mar;99(3):565–571. doi: 10.1111/j.1476-5381.1990.tb12969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Matsubara T., Nishizuka Y. Inhibitory action of guanosine 3', 5'-monophosphate on thrombin-induced phosphatidylinositol turnover and protein phosphorylation in human platelets. Biochem Biophys Res Commun. 1981 Jul 16;101(1):61–67. doi: 10.1016/s0006-291x(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]


